European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2018-01-12 , DOI: 10.1016/j.ejmech.2018.01.038 Wei-Wei Gao , Lavanya Gopala , Rammohan R. Yadav Bheemanaboina , Guo-Biao Zhang , Shuo Li , Cheng-He Zhou
|
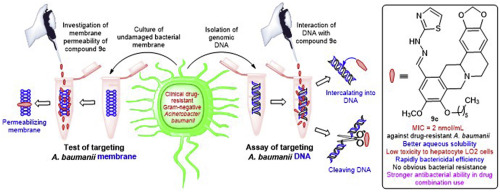
Aminothiazolyl berberine derivatives as potentially antimicrobial agents were designed and synthesized in an effort to overcome drug resistance. The antimicrobial assay revealed that some target compounds exhibited significantly inhibitory efficiencies toward bacteria and fungi including drug-resistant pathogens, and the aminothiazole and Schiff base moieties were helpful structural fragments for aqueous solubility and antibacterial activity. Especially, aminothiazolyl 9-hexyl berberine 9c and 2,4-dichlorobenzyl derivative 18a exhibited good activities (MIC = 2 nmol/mL) against clinically drug-resistant Gram-negative Acinetobacter baumanii with low cytotoxicity to hepatocyte LO2 cells, rapidly bactericidal effects and quite slow development of bacterial resistance toward A. baumanii. Molecular modeling indicated that compounds 9c and 18a could bind with GLY-102, ARG-136 and/or ALA-100 residues of DNA gyrase through hydrogen bonds. It was found that compounds 9c and 18a were able to disturb the drug-resistant A. baumanii membrane effectively, and molecule 9c could not only intercalate but also cleave bacterial DNA isolated from resistant A. baumanii, which might be the preliminary antibacterial action mechanism of inhibiting the growth of A. baumanii strain. In particular, the combination use of compound 9c with norfloxacin could enhance the antibacterial activity, broaden antibacterial spectrum and overcome the drug resistance.
中文翻译:

发现2-氨基噻唑基小ber碱衍生物作为对临床耐药的鲍氏不动杆菌革兰氏不动杆菌的有效抗菌剂
为了克服耐药性,设计并合成了氨基噻唑基小ber碱衍生物作为潜在的抗菌剂。抗菌测定表明,某些目标化合物对细菌和真菌(包括耐药病原体)表现出显着的抑制作用,而氨基噻唑和席夫碱部分是水溶性和抗菌活性的有用结构片段。特别是,氨基噻唑基9-己基小碱9c和2,4-二氯苄基衍生物18a对临床耐药的革兰氏阴性不动杆菌具有良好的活性(MIC = 2 nmol / mL)。对肝细胞LO2细胞具有较低的细胞毒性,具有快速的杀菌作用,并且对鲍曼不动杆菌的细菌耐药性发展相当缓慢。分子建模表明化合物9c和18a可以通过氢键与DNA促旋酶的GLY-102,ARG-136和/或ALA-100残基结合。发现化合物9c和18a能够有效地干扰耐药鲍曼不动杆菌膜,分子9c不仅可以插入而且裂解从耐药鲍曼不动杆菌中分离的细菌DNA ,这可能是鲍曼不动杆菌的初步抗菌作用机制。抑制鲍曼不动杆菌的生长拉紧。特别地,化合物9c与诺氟沙星的组合使用可增强抗菌活性,拓宽抗菌谱并克服耐药性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号