Tetrahedron ( IF 2.1 ) Pub Date : 2018-01-12 , DOI: 10.1016/j.tet.2017.12.049 Juan-Carlos Castillo , Elizabeth Jiménez , Jaime Portilla , Braulio Insuasty , Jairo Quiroga , Rodolfo Moreno-Fuquen , Alan R. Kennedy , Rodrigo Abonia
|
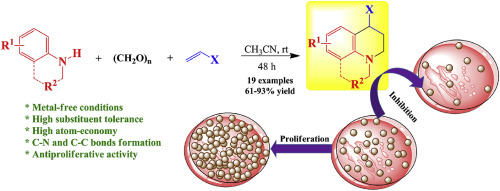
A useful and efficient method to construct diversely substituted 1,2,3,4-tetrahydroquinolines in good to excellent yields has been developed through a catalyst-free Domino Mannich and intramolecular Friedel-Crafts alkylation reactions of N-arylamines with paraformaldehyde and electron-rich olefins via the formation of N-aryl-N-alkylmethyleneiminium ions as the key intermediates to afford the target products. Nine of the new compounds were evaluated in the US National Cancer Institute (NCI), where compound 5f (R1 = 6-MeO, R2 = p-ClC6H4 and X = pyrrolidin-2-onyl) presented a remarkable activity against 57 cancer cell lines, with the most important GI50 values ranging from 1.46 to 8.28 μM from in vitro assays. Further studies performed over the active compound 5f on HCT116 colon cancer cells indicated that its effect on cell death is exerted through a cell cycle arrest (S phase) in a dose dependent manner, as well as suppression on the cell proliferation process.
中文翻译:

无催化剂的Domino Mannich / Friedel-Crafts烷基化反应在合成具有潜在抗肿瘤活性的新型四氢喹啉中的应用
通过无催化剂的多米诺·曼尼希(Domino Mannich)和N-芳基胺与多聚甲醛和富电子的分子内Friedel-Crafts烷基化反应,已开发出一种有用且有效的方法,以良好的优良率构建各种取代的1,2,3,4-四氢喹啉。烯烃通过形成ñ -芳基- ñ -alkylmethyleneiminium离子作为关键中间体,得到目标产物。在美国国家癌症研究所(NCI)中评估了九种新化合物,其中化合物5f(R1 = 6-MeO,R2 = p -ClC6H4和X =吡咯烷-2-烯丙基)对57种癌细胞系表现出显着活性,最重要的GI50值范围为1.46至8.28μM体外测定。在HCT116结肠癌细胞上对活性化合物5f进行的进一步研究表明,其对细胞死亡的作用是通过细胞周期停滞(S期)以剂量依赖性方式发挥的,以及对细胞增殖过程的抑制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号