当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Isomerism and reactivity of nickel(ii) acetylacetonate bis(thiosemicarbazone) complexes†
Dalton Transactions ( IF 4 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1039/c7dt04337f Jessica K. Bilyj 1, 2, 3, 4 , Mark J. Riley 1, 2, 3, 4 , Paul V. Bernhardt 1, 2, 3, 4
Dalton Transactions ( IF 4 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1039/c7dt04337f Jessica K. Bilyj 1, 2, 3, 4 , Mark J. Riley 1, 2, 3, 4 , Paul V. Bernhardt 1, 2, 3, 4
Affiliation
|
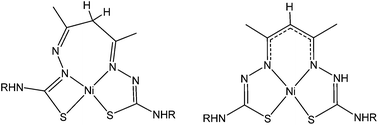
The complexation of nickel(II) with acetylacetonate bis(thiosemicarbazone) N2S2 ligands with varying substituents has revealed that two isomers can exist independently in solution. These isomers differ according to the formation of either a 5,6,5-membered (symmetric) or a 4,7,5-membered (asymmetric) chelate ring arrangement. These two isomers have distinctly different properties. The symmetric complex (sym-[Ni(acacR)]) is unstable in the presence of air and slowly converts to the oxidised analogue sym-[Ni(acacRO)] with a carbonyl group installed at the apical C-atom. The mechanism of this O-atom transfer reaction is still unclear but kinetic and spectroelectrochemical experiments in addition to Density Functional Theory calculations have identified a single electron oxidised NiII–ligand radical complex as a key intermediate. By contrast the asymmetric complex, asym-[Ni(acacR)] is inert to ligand oxidation.
中文翻译:

乙酰丙酮 镍(ii)双(硫代半碳carb酮)配合物的异构性和反应性†
镍(II)与具有不同取代基的乙酰丙酮双(硫代半脲)N 2 S 2配体的络合表明,溶液中可以独立存在两种异构体。这些异构体根据5,6,5-元(对称)或4,7,5-元(不对称)螯合环排列的形成而不同。这两种异构体具有明显不同的性质。对称复合物(符号- [镍(acacR)])是在空气中和慢慢地转换的存在下被氧化的类似物不稳定符号-[Ni(acacRO)],在顶端C原子处安装有羰基。这种O原子转移反应的机理尚不清楚,但除密度泛函理论计算外,动力学和光谱电化学实验还确定了单电子氧化的Ni II-配体自由基络合物为关键中间体。相比之下,不对称络合物,ASYM - [镍(acacR)]是惰性的配位体的氧化。
更新日期:2018-01-12
中文翻译:

乙酰丙酮 镍(ii)双(硫代半碳carb酮)配合物的异构性和反应性†
镍(II)与具有不同取代基的乙酰丙酮双(硫代半脲)N 2 S 2配体的络合表明,溶液中可以独立存在两种异构体。这些异构体根据5,6,5-元(对称)或4,7,5-元(不对称)螯合环排列的形成而不同。这两种异构体具有明显不同的性质。对称复合物(符号- [镍(acacR)])是在空气中和慢慢地转换的存在下被氧化的类似物不稳定符号-[Ni(acacRO)],在顶端C原子处安装有羰基。这种O原子转移反应的机理尚不清楚,但除密度泛函理论计算外,动力学和光谱电化学实验还确定了单电子氧化的Ni II-配体自由基络合物为关键中间体。相比之下,不对称络合物,ASYM - [镍(acacR)]是惰性的配位体的氧化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号