当前位置:
X-MOL 学术
›
Chem. Eng. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanistic and constrained thermochemical modelling in chemical reactor engineering: Ti(IV)Chloride oxidation revisited
Chemical Engineering Science ( IF 4.7 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.ces.2018.01.016 Pertti Koukkari , Eduardo Paiva
Chemical Engineering Science ( IF 4.7 ) Pub Date : 2018-04-01 , DOI: 10.1016/j.ces.2018.01.016 Pertti Koukkari , Eduardo Paiva
|
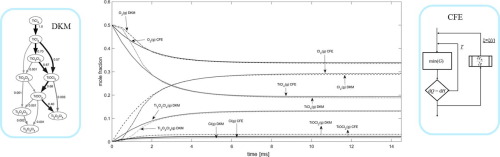
Abstract In process and materials chemistry, computational modelling of complex reactive systems has been a long-time continuing process. The methodology based on numerical methods in mechanistic reaction kinetics as well as for fluid phase thermodynamics applying equations of state is well established. During the last two decades, however, thermodynamic multiphase technology based on the minimisation of Gibbs free energy has made progress in modelling both reactive flows and processing of functional materials. Recent advancements also include introduction of such new Gibbs’ian algorithms, which facilitate calculation of time-dependent changes in reactive multi-component multi-phase systems. Comparison between detailed kinetic mechanisms (DKMs) and multiphase thermochemical techniques has yet seldom been available. The present work describes a unique comparison between constrained Gibbs free energy calculation (with one constrained kinetic reaction rate) and detailed kinetic mechanism of 67 reversible reactions between 28 species, as performed for the industrially interesting Titanium(IV) chloride oxidation process. When confined to gas phase kinetics, the calculated results show fair agreement between the two different techniques. However, comparison with observations from an experimental plug flow reactor suggests that the formation of a solid Ti-oxide phase in the models becomes a necessity. The applicability of the alternative simulation techniques in chemical reactor engineering is shortly discussed.
中文翻译:

化学反应器工程中的机械和约束热化学建模:重新审视氯化钛氧化
摘要 在过程和材料化学中,复杂反应系统的计算建模是一个长期持续的过程。基于机械反应动力学中的数值方法以及应用状态方程的流体相热力学的方法已经很好地建立。然而,在过去的二十年中,基于吉布斯自由能最小化的热力学多相技术在模拟反应流和功能材料加工方面取得了进展。最近的进展还包括引入了这种新的吉布斯算法,这有助于计算反应性多分量多相系统中的时间相关变化。详细动力学机制 (DKM) 和多相热化学技术之间的比较很少可用。目前的工作描述了受限吉布斯自由能计算(具有一个受限动力学反应速率)与 28 种物质之间 67 种可逆反应的详细动力学机制之间的独特比较,如工业上有趣的氯化钛 (IV) 氧化过程所执行的。当仅限于气相动力学时,计算结果显示两种不同技术之间的一致性。然而,与来自实验性活塞流反应器的观察结果进行比较表明,在模型中形成固体钛氧化物相变得必要。简要讨论了替代模拟技术在化学反应器工程中的适用性。
更新日期:2018-04-01
中文翻译:

化学反应器工程中的机械和约束热化学建模:重新审视氯化钛氧化
摘要 在过程和材料化学中,复杂反应系统的计算建模是一个长期持续的过程。基于机械反应动力学中的数值方法以及应用状态方程的流体相热力学的方法已经很好地建立。然而,在过去的二十年中,基于吉布斯自由能最小化的热力学多相技术在模拟反应流和功能材料加工方面取得了进展。最近的进展还包括引入了这种新的吉布斯算法,这有助于计算反应性多分量多相系统中的时间相关变化。详细动力学机制 (DKM) 和多相热化学技术之间的比较很少可用。目前的工作描述了受限吉布斯自由能计算(具有一个受限动力学反应速率)与 28 种物质之间 67 种可逆反应的详细动力学机制之间的独特比较,如工业上有趣的氯化钛 (IV) 氧化过程所执行的。当仅限于气相动力学时,计算结果显示两种不同技术之间的一致性。然而,与来自实验性活塞流反应器的观察结果进行比较表明,在模型中形成固体钛氧化物相变得必要。简要讨论了替代模拟技术在化学反应器工程中的适用性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号