Synthesis ( IF 2.6 ) Pub Date : 2018-01-11 , DOI: 10.1055/s-0036-1591871 Lluis Marsal 1 , Max von Delius 2 , Michael Bothe 2 , María Montero-Rama 1 , Aurélien Viterisi 1 , Werther Cambarau 3 , Caterina Stenta 1 , Emilio Palomares 3
|
Published as part of the Bürgenstock Special Section 2017 Future Stars in Organic Chemistry
Abstract
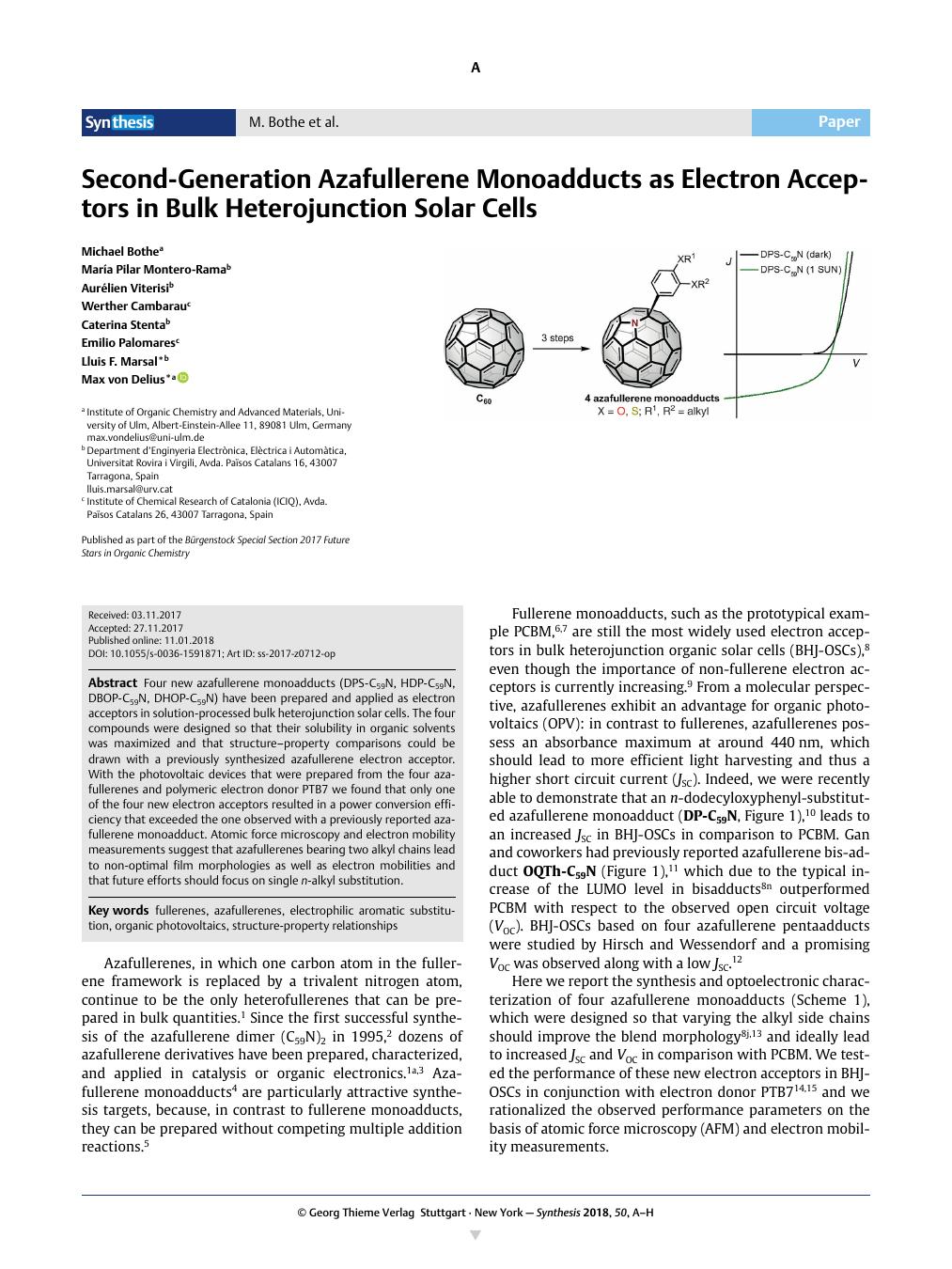
Four new azafullerene monoadducts (DPS-C59N, HDP-C59N, DBOP-C59N, DHOP-C59N) have been prepared and applied as electron acceptors in solution-processed bulk heterojunction solar cells. The four compounds were designed so that their solubility in organic solvents was maximized and that structure–property comparisons could be drawn with a previously synthesized azafullerene electron acceptor. With the photovoltaic devices that were prepared from the four azafullerenes and polymeric electron donor PTB7 we found that only one of the four new electron acceptors resulted in a power conversion efficiency that exceeded the one observed with a previously reported azafullerene monoadduct. Atomic force microscopy and electron mobility measurements suggest that azafullerenes bearing two alkyl chains lead to non-optimal film morphologies as well as electron mobilities and that future efforts should focus on single n-alkyl substitution.
Four new azafullerene monoadducts (DPS-C59N, HDP-C59N, DBOP-C59N, DHOP-C59N) have been prepared and applied as electron acceptors in solution-processed bulk heterojunction solar cells. The four compounds were designed so that their solubility in organic solvents was maximized and that structure–property comparisons could be drawn with a previously synthesized azafullerene electron acceptor. With the photovoltaic devices that were prepared from the four azafullerenes and polymeric electron donor PTB7 we found that only one of the four new electron acceptors resulted in a power conversion efficiency that exceeded the one observed with a previously reported azafullerene monoadduct. Atomic force microscopy and electron mobility measurements suggest that azafullerenes bearing two alkyl chains lead to non-optimal film morphologies as well as electron mobilities and that future efforts should focus on single n-alkyl substitution.
中文翻译:

第二代氮杂富勒烯单加合物作为体异质结太阳能电池中的电子受体
作为Bürgenstock专栏2017年有机化学中的未来之星的一部分发布
抽象的
四个新的氮杂富勒烯单加合物(DPS-C 59 N,HDP-C 59 N,DBOP-C 59 N,DHOP-C 59N)已经被制备并用作溶液处理的整体异质结太阳能电池中的电子受体。对这四种化合物进行了设计,以使其在有机溶剂中的溶解度最大化,并可以使用先前合成的氮杂富勒烯电子受体进行结构-性能比较。用由四个氮杂富勒烯和聚合物电子给体PTB7制备的光伏器件,我们发现四个新的电子受体中只有一个产生的功率转换效率超过了先前报道的氮杂富勒烯单加合物所观察到的功率转换效率。原子力显微镜和电子迁移率测量表明,带有两个烷基链的氮杂富勒烯导致非最佳的膜形态和电子迁移率,未来的研究应集中在单一正烷基取代。
四个新的氮杂富勒烯单加合物(DPS-C 59 N,HDP-C 59 N,DBOP-C 59 N,DHOP-C 59N)已经被制备并用作溶液处理的整体异质结太阳能电池中的电子受体。对这四种化合物进行了设计,以使其在有机溶剂中的溶解度最大化,并可以使用先前合成的氮杂富勒烯电子受体进行结构-性能比较。用由四个氮杂富勒烯和聚合物电子给体PTB7制备的光伏器件,我们发现四个新的电子受体中只有一个产生的功率转换效率超过了先前报道的氮杂富勒烯单加合物所观察到的功率转换效率。原子力显微镜和电子迁移率测量表明,带有两个烷基链的氮杂富勒烯导致非最佳的膜形态和电子迁移率,未来的研究应集中在单一正烷基取代。


























 京公网安备 11010802027423号
京公网安备 11010802027423号