当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Drastic Redox Shift and Electronic Structural Changes of a Manganese(III)-Salen Oxidation Catalyst upon Reaction with Hydroxide and Cyanide Ion
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02474 Takuya Kurahashi 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02474 Takuya Kurahashi 1
Affiliation
|
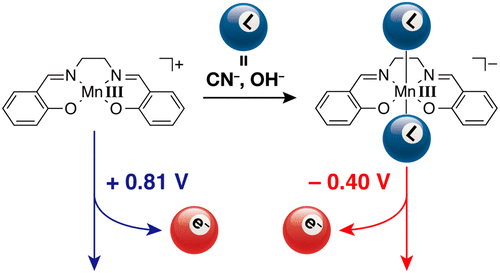
Flexible redox properties of a metal complex are important for redox catalysis. The present study shows that the reaction of a manganese(III) salen complex, which is a well-known oxidation catalyst, with hydroxide ion gives a transient manganese(III) species with drastically lowered redox potential, where the redox difference is −1.21 V. The reaction with cyanide ion gives a stable manganese(III) species with almost the same spectroscopic and redox properties, which was characterized as an anionic [MnIII(salen)(CN)2]− of low-spin S = 1 state, in contrast to the starting MnIII(salen)(OTf) having usual high-spin S = 2 manganese(III). The present study has thus clarified that the drastic redox shift comes from an anionic six-coordinate [MnIII(salen)(X)2]− species where X is either OH– or CN–. Resonance Raman measurements show that the stretching band of the imino group shifts from 1620 to 1597 cm–1 upon conversion from MnIII(salen)(OTf) to [MnIII(salen)(CN)2]−, indicative of lowered C═N double bond character for [MnIII(salen)(CN)2]−. The observed deformation of a salen ligand is a clear indication of an increased electron population on the imino π*-orbital upon formation of low-spin manganese(III). It was proposed that the electronic structure of [MnIII(salen)(CN)2]− may contain only limited contribution from valence tautomeric [MnIV(salen– •)(CN)2]−, in which the imino group of a salen ligand is reduced by one-electron via intramolecular electron transfer from low-spin manganese(III). The present study has clarified an unexpected new finding that a salen ligand works as a reservoir for negative charge to stabilize low-spin manganese(III).
中文翻译:

氢氧化物与氰化物离子反应后锰(III)-萨伦氧化催化剂的剧烈氧化还原位移和电子结构变化
金属配合物的柔性氧化还原性质对于氧化还原催化很重要。本研究表明,锰(III)塞伦配合物(一种众所周知的氧化催化剂)与氢氧根离子的反应产生了一种瞬态锰(III),其氧化还原电位显着降低,其中氧化还原差为-1.21 V 。与氰化物离子的反应产生了稳定的锰(III)物种,具有几乎相同的光谱和氧化还原特性,其特征为低旋转S = 1态的阴离子[Mn III(salen)(CN)2 ] -,与通常具有高自旋S的起始Mn III(salen)(OTf)相比= 2锰(III)。因此,本研究已表明的急剧氧化还原移来自阴离子的六坐标[锰III(沙仑)(X)2 ] -物种,其中X是OH -或CN - 。共振拉曼测量表明,亚氨基的拉伸带在从Mn III(salen)(OTf)转变为[Mn III(salen)(CN)2 ] -时从1620变为1597 cm -1,表明C═降低。 [Mn III(salen)(CN)2 ]的N双键特征-。观察到的Salen配体变形清楚地表明在形成低自旋锰(III)时亚氨基π*轨道上的电子数量增加。有人提出[Mn III(salen)(CN)2 ] -的电子结构可能只包含价互变异构体[Mn IV(salen- •)(CN)2 ] -的有限贡献,其中a的亚氨基通过低自旋锰(III)的分子内电子转移,salen配体被单电子还原。本研究澄清了一个出乎意料的新发现,即Salen配体可充当负电荷的储库,以稳定低旋转锰(III)。
更新日期:2018-01-11
中文翻译:

氢氧化物与氰化物离子反应后锰(III)-萨伦氧化催化剂的剧烈氧化还原位移和电子结构变化
金属配合物的柔性氧化还原性质对于氧化还原催化很重要。本研究表明,锰(III)塞伦配合物(一种众所周知的氧化催化剂)与氢氧根离子的反应产生了一种瞬态锰(III),其氧化还原电位显着降低,其中氧化还原差为-1.21 V 。与氰化物离子的反应产生了稳定的锰(III)物种,具有几乎相同的光谱和氧化还原特性,其特征为低旋转S = 1态的阴离子[Mn III(salen)(CN)2 ] -,与通常具有高自旋S的起始Mn III(salen)(OTf)相比= 2锰(III)。因此,本研究已表明的急剧氧化还原移来自阴离子的六坐标[锰III(沙仑)(X)2 ] -物种,其中X是OH -或CN - 。共振拉曼测量表明,亚氨基的拉伸带在从Mn III(salen)(OTf)转变为[Mn III(salen)(CN)2 ] -时从1620变为1597 cm -1,表明C═降低。 [Mn III(salen)(CN)2 ]的N双键特征-。观察到的Salen配体变形清楚地表明在形成低自旋锰(III)时亚氨基π*轨道上的电子数量增加。有人提出[Mn III(salen)(CN)2 ] -的电子结构可能只包含价互变异构体[Mn IV(salen- •)(CN)2 ] -的有限贡献,其中a的亚氨基通过低自旋锰(III)的分子内电子转移,salen配体被单电子还原。本研究澄清了一个出乎意料的新发现,即Salen配体可充当负电荷的储库,以稳定低旋转锰(III)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号