当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modular [FeIII8MII6]n+ (MII = Pd, Co, Ni, Cu) Coordination Cages
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02674 Sergio Sanz 1 , Helen M. O’Connor 1 , Priyanka Comar 1 , Amgalanbaatar Baldansuren 2 , Mateusz B. Pitak 3 , Simon J. Coles 3 , Høgni Weihe 4 , Nicholas F. Chilton 2 , Eric J. L. McInnes 2 , Paul J. Lusby 1 , Stergios Piligkos 4 , Euan K. Brechin 1
Inorganic Chemistry ( IF 4.6 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02674 Sergio Sanz 1 , Helen M. O’Connor 1 , Priyanka Comar 1 , Amgalanbaatar Baldansuren 2 , Mateusz B. Pitak 3 , Simon J. Coles 3 , Høgni Weihe 4 , Nicholas F. Chilton 2 , Eric J. L. McInnes 2 , Paul J. Lusby 1 , Stergios Piligkos 4 , Euan K. Brechin 1
Affiliation
|
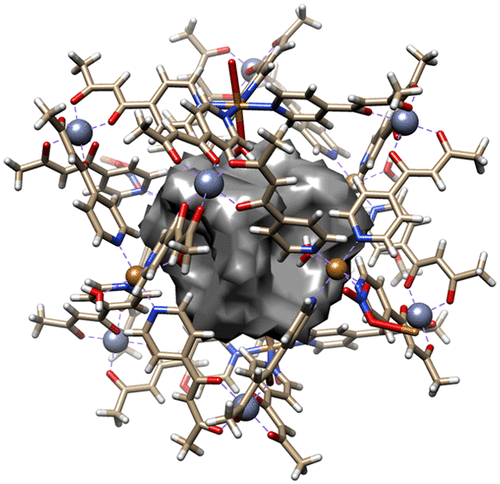
The reaction of the simple metalloligand [FeIIIL3] [HL = 1-(4-pyridyl)butane-1,3-dione] with a variety of different MII salts results in the formation of a family of heterometallic cages of formulae [FeIII8PdII6L24]Cl12 (1), [FeIII8CuII6L24(H2O)4Br4]Br8 (2), [FeIII8CuII6L24(H2O)10](NO3)12 (3), [FeIII8NiII6L24(SCN)11Cl] (4), and [FeIII8CoII6L24(SCN)10(H2O)2]Cl2 (5). The metallic skeleton of each cage describes a cube in which the FeIII ions occupy the eight vertices and the MII ions lie at the center of the six faces. Direct-current magnetic susceptibility and magnetization measurements on 3–5 reveal the presence of weak antiferromagnetic exchange between the metal ions in all three cases. Computational techniques known in theoretical nuclear physics as statistical spectroscopy, which exploit the moments of the Hamiltonian to calculate relevant thermodynamic properties, determine JFe–Cu = 0.10 cm–1 for 3 and JFe–Ni = 0.025 cm–1 for 4. Q-band electron paramagnetic resonance spectra of 1 reveal a significantly wider spectral width in comparison to [FeL3], indicating that the magnitude of the FeIII zero-field splitting is larger in the heterometallic cage than in the monomer.
中文翻译:

模块化[Fe III 8 M II 6 ] n +(M II = Pd,Co,Ni,Cu)配位笼
简单的金属配体[Fe III L 3 ] [HL = 1-(4-吡啶基)丁烷-1,3-二酮]与多种不同的M II盐的反应导致形成下式的杂金属笼族[Fe III 8 Pd II 6 L 24 ] Cl 12(1),[Fe III 8 Cu II 6 L 24(H 2 O)4 Br 4 ] Br 8(2),[Fe III 8 Cu II 6 L 24(H 2 O)10 ](NO 3)12(3),[Fe III 8 Ni II 6 L 24(SCN)11 Cl](4)和[Fe III 8 Co II 6 L 24(SCN)10( H 2 O)2 ] Cl 2(5)。每个笼子的金属骨架都描述了一个立方体,其中Fe III离子占据了八个顶点,而M II离子位于六个面的中心。在3 – 5上的直流磁化率和磁化强度测量表明,在所有三种情况下,金属离子之间都存在弱的反铁磁交换。在理论核物理学作为统计光谱,其中利用哈密顿的时刻,以计算有关的热力学性质已知的计算技术,确定Ĵ铁铜=0.10厘米-1为3和Ĵ的Fe-Ni =0.025厘米-1为4。Q带电子顺磁共振光谱1相比于[FEL揭示显著更宽的光谱宽度由图3可知,Fe III零场分裂的大小在杂金属笼中比在单体中大。
更新日期:2018-01-11
中文翻译:

模块化[Fe III 8 M II 6 ] n +(M II = Pd,Co,Ni,Cu)配位笼
简单的金属配体[Fe III L 3 ] [HL = 1-(4-吡啶基)丁烷-1,3-二酮]与多种不同的M II盐的反应导致形成下式的杂金属笼族[Fe III 8 Pd II 6 L 24 ] Cl 12(1),[Fe III 8 Cu II 6 L 24(H 2 O)4 Br 4 ] Br 8(2),[Fe III 8 Cu II 6 L 24(H 2 O)10 ](NO 3)12(3),[Fe III 8 Ni II 6 L 24(SCN)11 Cl](4)和[Fe III 8 Co II 6 L 24(SCN)10( H 2 O)2 ] Cl 2(5)。每个笼子的金属骨架都描述了一个立方体,其中Fe III离子占据了八个顶点,而M II离子位于六个面的中心。在3 – 5上的直流磁化率和磁化强度测量表明,在所有三种情况下,金属离子之间都存在弱的反铁磁交换。在理论核物理学作为统计光谱,其中利用哈密顿的时刻,以计算有关的热力学性质已知的计算技术,确定Ĵ铁铜=0.10厘米-1为3和Ĵ的Fe-Ni =0.025厘米-1为4。Q带电子顺磁共振光谱1相比于[FEL揭示显著更宽的光谱宽度由图3可知,Fe III零场分裂的大小在杂金属笼中比在单体中大。



























 京公网安备 11010802027423号
京公网安备 11010802027423号