当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Potassium associated manganese vacancy in birnessite-type manganese dioxide for airborne formaldehyde oxidation†
Catalysis Science & Technology ( IF 5 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1039/c7cy02121f Shaopeng Rong 1, 2, 3, 4, 5 , Kezhi Li 1, 2, 3, 4, 5 , Pengyi Zhang 1, 2, 3, 4, 5 , Fang Liu 1, 2, 3, 4, 5 , Junying Zhang 6, 7, 8, 9
Catalysis Science & Technology ( IF 5 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1039/c7cy02121f Shaopeng Rong 1, 2, 3, 4, 5 , Kezhi Li 1, 2, 3, 4, 5 , Pengyi Zhang 1, 2, 3, 4, 5 , Fang Liu 1, 2, 3, 4, 5 , Junying Zhang 6, 7, 8, 9
Affiliation
|
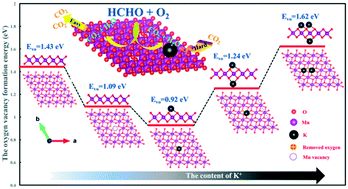
As a strategy for regulating the electronic structure of metal oxides, defect engineering has been widely studied, and the concentrations and spatial distributions of metal vacancies in metal oxides have always resulted in unprecedented properties. Moreover, alkali metals exhibit a universal promotion effect on catalytic oxidation of formaldehyde (HCHO). Herein, a kind of birnessite-type manganese dioxide (MnO2) with many Mn vacancies was hydrothermally synthesized for catalytic oxidation of HCHO. The significant effect of the K+ content on the structure, morphology and catalytic activity of birnessite-type MnO2 for HCHO oxidation was systematically studied for the first time. Initially, the increasing content of K+ obviously improved the catalytic performance for HCHO oxidation due to the considerable enhancement of the lattice oxygen activity. However, due to interaction with the excess K atoms, the oxygen atoms nearest to the K atoms were more stable and their mobility decreased, which was confirmed by experimental characterization and DFT (density functional theory) calculation. Moreover, the excess K+ increased the amount of surface basic sites, making CO2 difficult to desorb. Thus, there was an optimal K+ content to promote the activity of birnessite-type MnO2. With moderate K+ content in the birnessite-type MnO2, excellent catalytic activity for HCHO oxidation was achieved (T50% = 56 °C; T90% = 82 °C) under 100 ppm of HCHO and ∼90 L gcat−1 h−1 of gas hourly space velocity (GHSV). The present work provided an insight into the structure–activity relationship between birnessite-type MnO2 and its catalytic activity.
中文翻译:

水钠锰矿型二氧化锰中钾相关的锰空位用于空气中甲醛氧化†
作为调节金属氧化物的电子结构的策略,缺陷工程已被广泛研究,并且金属空位在金属氧化物中的浓度和空间分布一直导致空前的性能。此外,碱金属对甲醛(HCHO)的催化氧化具有普遍的促进作用。在此,水热合成了一种具有许多Mn空位的水钠锰矿型二氧化锰(MnO 2),用于催化氧化HCHO。首次系统地研究了K +含量对水钠锰矿型MnO 2的结构,形貌和催化活性的显着影响。最初,K +的含量不断增加由于晶格氧活性的显着提高,明显改善了HCHO氧化的催化性能。然而,由于与过量的K原子相互作用,最接近K原子的氧原子更加稳定,其迁移率下降,这已通过实验表征和DFT(密度泛函理论)计算得到证实。而且,过量的K +增加了表面碱性位点的数量,使得CO 2难以解吸。因此,存在最佳的K +含量以促进水钠锰矿型MnO 2的活性。在水钠锰矿型MnO 2中具有适度的K +含量,实现了对HCHO氧化的优异催化活性(T 50% = 56°C; 在100 ppm的HCHO和约90 L g cat -1 h -1的气体时空速(GHSV)下,T 90% = 82°C 。目前的工作提供了对水钠锰矿型MnO 2和其催化活性之间的结构活性关系的见解。
更新日期:2018-01-11
中文翻译:

水钠锰矿型二氧化锰中钾相关的锰空位用于空气中甲醛氧化†
作为调节金属氧化物的电子结构的策略,缺陷工程已被广泛研究,并且金属空位在金属氧化物中的浓度和空间分布一直导致空前的性能。此外,碱金属对甲醛(HCHO)的催化氧化具有普遍的促进作用。在此,水热合成了一种具有许多Mn空位的水钠锰矿型二氧化锰(MnO 2),用于催化氧化HCHO。首次系统地研究了K +含量对水钠锰矿型MnO 2的结构,形貌和催化活性的显着影响。最初,K +的含量不断增加由于晶格氧活性的显着提高,明显改善了HCHO氧化的催化性能。然而,由于与过量的K原子相互作用,最接近K原子的氧原子更加稳定,其迁移率下降,这已通过实验表征和DFT(密度泛函理论)计算得到证实。而且,过量的K +增加了表面碱性位点的数量,使得CO 2难以解吸。因此,存在最佳的K +含量以促进水钠锰矿型MnO 2的活性。在水钠锰矿型MnO 2中具有适度的K +含量,实现了对HCHO氧化的优异催化活性(T 50% = 56°C; 在100 ppm的HCHO和约90 L g cat -1 h -1的气体时空速(GHSV)下,T 90% = 82°C 。目前的工作提供了对水钠锰矿型MnO 2和其催化活性之间的结构活性关系的见解。



























 京公网安备 11010802027423号
京公网安备 11010802027423号