当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photo-triggered micelles: simultaneous activation and release of microtubule inhibitors for on-demand chemotherapy†
Biomaterials Science ( IF 6.6 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1039/c7bm01053b Chao Chen 1, 2, 3, 4, 5 , Jie Zhao 1, 2, 3, 4, 5 , Min Gao 1, 2, 3, 4, 5 , Xuan Meng 1, 2, 3, 4, 5 , Aiping Fan 1, 2, 3, 4, 5 , Zheng Wang 1, 2, 3, 4, 5 , Yanjun Zhao 1, 2, 3, 4, 5
Biomaterials Science ( IF 6.6 ) Pub Date : 2018-01-11 00:00:00 , DOI: 10.1039/c7bm01053b Chao Chen 1, 2, 3, 4, 5 , Jie Zhao 1, 2, 3, 4, 5 , Min Gao 1, 2, 3, 4, 5 , Xuan Meng 1, 2, 3, 4, 5 , Aiping Fan 1, 2, 3, 4, 5 , Zheng Wang 1, 2, 3, 4, 5 , Yanjun Zhao 1, 2, 3, 4, 5
Affiliation
|
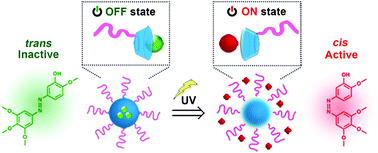
The nonspecific biodistribution of cytotoxic drugs and associated adverse effects greatly limit the efficacy and patient compliance of chemotherapy. To address this, we employed a photoswitchable microtubule inhibitor (Azo-CA4) that was physically loaded in cyclodextrin-bearing micellar nanocarriers through the host–guest interaction. Azo-CA4 was only activated upon ultraviolet (UV) light irradiation to trigger the transition from the “trans” (inactive) to “cis” (active) state. Such conformation change could then induce rapid Azo-CA4 release from micelles without the delay of the onset of therapeutic action. This nanoscale delivery system produced photo-triggered antimitotic and pro-apoptotic effects in MDA-MB-231 cells via a triggered control of microtubule dynamics. The anticancer efficacy of Azo-CA4-loaded micelles was further proved in vivo using a 4T1 tumor-bearing mice model coupled with multiple topical administrations to avoid the penetration problem of UV light. This work provides a new delivery vehicle to aid the application and potential translation of Azo-CA4 as biomedical tools and precision chemotherapeutics.
中文翻译:

光触发的胶束:同时激活和释放用于按需化学疗法的微管抑制剂†
细胞毒性药物的非特异性生物分布以及相关的不良反应极大地限制了化疗的疗效和患者的依从性。为了解决这个问题,我们采用了一种可光转换的微管抑制剂(Azo-CA4),该抑制剂通过宿主与客体的相互作用而物理负载在带有环糊精的胶束纳米载体中。Azo-CA4仅在紫外线(UV)照射下被激活,以触发从“反式”(非活性)到“顺式”(活性)状态的转变。然后,这种构象变化可以诱导从胶束中快速释放Azo-CA4,而不会延迟治疗作用的发作。该纳米级递送系统通过以下途径在MDA-MB-231细胞中产生了光触发的抗有丝分裂和促凋亡作用微管动力学的触发控制。载有偶氮-CA4的胶束的抗癌功效在体内通过使用带有4T1肿瘤的小鼠模型和多次局部给药避免了紫外线的渗透问题得到了进一步证明。这项工作提供了一种新的载体,可帮助将Azo-CA4用作生物医学工具和精密化学疗法并进行潜在的翻译。
更新日期:2018-01-11
中文翻译:

光触发的胶束:同时激活和释放用于按需化学疗法的微管抑制剂†
细胞毒性药物的非特异性生物分布以及相关的不良反应极大地限制了化疗的疗效和患者的依从性。为了解决这个问题,我们采用了一种可光转换的微管抑制剂(Azo-CA4),该抑制剂通过宿主与客体的相互作用而物理负载在带有环糊精的胶束纳米载体中。Azo-CA4仅在紫外线(UV)照射下被激活,以触发从“反式”(非活性)到“顺式”(活性)状态的转变。然后,这种构象变化可以诱导从胶束中快速释放Azo-CA4,而不会延迟治疗作用的发作。该纳米级递送系统通过以下途径在MDA-MB-231细胞中产生了光触发的抗有丝分裂和促凋亡作用微管动力学的触发控制。载有偶氮-CA4的胶束的抗癌功效在体内通过使用带有4T1肿瘤的小鼠模型和多次局部给药避免了紫外线的渗透问题得到了进一步证明。这项工作提供了一种新的载体,可帮助将Azo-CA4用作生物医学工具和精密化学疗法并进行潜在的翻译。


























 京公网安备 11010802027423号
京公网安备 11010802027423号