当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Activity-Related Microsecond Dynamics Revealed by Temperature-Jump Förster Resonance Energy Transfer Measurements on Thermophilic Alcohol Dehydrogenase
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-01-11 , DOI: 10.1021/jacs.7b12369 Morgan B. Vaughn 1 , Jianyu Zhang , Thomas G. Spiro 2 , R. Brian Dyer 1 , Judith P. Klinman
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-01-11 , DOI: 10.1021/jacs.7b12369 Morgan B. Vaughn 1 , Jianyu Zhang , Thomas G. Spiro 2 , R. Brian Dyer 1 , Judith P. Klinman
Affiliation
|
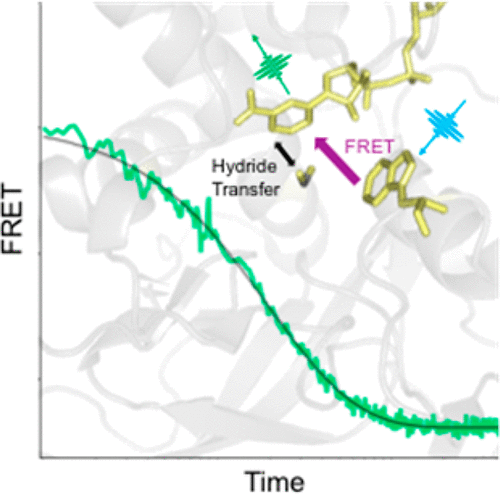
Previous studies of a thermophilic alcohol dehydrogenase (ht-ADH) demonstrated a range of discontinuous transitions at 30 °C that include catalysis, kinetic isotope effects, protein hydrogen-deuterium exchange rates, and intrinsic fluorescence properties. Using the Förster resonance energy transfer response from a Trp-NADH donor-acceptor pair in T-jump studies of ht-ADH, we now report microsecond protein motions that can be directly related to active site chemistry. Two distinctive transients are observed: a slow, kinetic process lacking a temperature break, together with a faster transient that is only detectable above 30 °C. The latter establishes a link between enzyme activity and microsecond protein motions near the cofactor binding site, in a region distinct from a previously detected protein network that communicates with the substrate binding site. Though evidence of direct dynamical links between microsecond protein motions and active site bond cleavage events is extremely rare, these studies highlight the potential of T-jump measurements to uncover such properties.
中文翻译:

通过对嗜热醇脱氢酶的温度跳跃 Förster 共振能量转移测量揭示与活动相关的微秒动力学
先前对嗜热醇脱氢酶 (ht-ADH) 的研究表明,在 30 °C 下会发生一系列不连续转变,包括催化、动力学同位素效应、蛋白质氢-氘交换率和内在荧光特性。在 ht-ADH 的 T 跳跃研究中,使用 Trp-NADH 供体-受体对的 Förster 共振能量转移响应,我们现在报告可以与活性位点化学直接相关的微秒蛋白质运动。观察到两个独特的瞬变:一个缓慢的动力学过程,没有温度中断,以及一个更快的瞬变,只有在 30°C 以上才能检测到。后者在辅因子结合位点附近建立了酶活性和微秒蛋白质运动之间的联系,在与先前检测到的与底物结合位点连通的蛋白质网络不同的区域中。尽管微秒蛋白质运动与活性位点键裂解事件之间存在直接动力学联系的证据极为罕见,但这些研究强调了 T 跳跃测量揭示此类特性的潜力。
更新日期:2018-01-11
中文翻译:

通过对嗜热醇脱氢酶的温度跳跃 Förster 共振能量转移测量揭示与活动相关的微秒动力学
先前对嗜热醇脱氢酶 (ht-ADH) 的研究表明,在 30 °C 下会发生一系列不连续转变,包括催化、动力学同位素效应、蛋白质氢-氘交换率和内在荧光特性。在 ht-ADH 的 T 跳跃研究中,使用 Trp-NADH 供体-受体对的 Förster 共振能量转移响应,我们现在报告可以与活性位点化学直接相关的微秒蛋白质运动。观察到两个独特的瞬变:一个缓慢的动力学过程,没有温度中断,以及一个更快的瞬变,只有在 30°C 以上才能检测到。后者在辅因子结合位点附近建立了酶活性和微秒蛋白质运动之间的联系,在与先前检测到的与底物结合位点连通的蛋白质网络不同的区域中。尽管微秒蛋白质运动与活性位点键裂解事件之间存在直接动力学联系的证据极为罕见,但这些研究强调了 T 跳跃测量揭示此类特性的潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号