Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2018-01-11 , DOI: 10.1016/j.bioorg.2018.01.007 Garima Verma , Gousia Chashoo , Asif Ali , Mohemmed Faraz Khan , Wasim Akhtar , Israr Ali , Mymoona Akhtar , Mohammad Mumtaz Alam , Mohammad Shaquiquzzaman
|
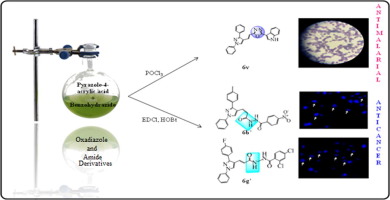
Depravity of malaria in terms of morbidity and mortality in human beings makes it a major health issue in tropical and subtropical areas of the globe. Drug counterfeiting and non-adherence to the treatment regimen have significantly contributed to development and spread of multidrug resistance that has highlighted the need for development of novel and more efficient antimalarial drugs. Complexity associated with cancer disease and prevalence of diversified cell populations vindicates highly specific treatment options for treatment of cancer. Resistance to these anticancer agents has posed a great hindrance in successful treatment of cancer. Pondering this ongoing situation, it was speculated to develop novel compounds targeting malaria and cancer. Moving on the same aisle, we synthesized pyrazole acrylic acid based oxadiazole and amide derivatives using multi-step reaction pathways (6a–x; 6a′–h′). Schizont maturation inhibition assay was employed to determine antimalarial potential. Compound 6v emerged as the most potent antimalarial agent targeting falcipain-2 enzyme. Anticancer activity was done using sulforhodamine B assay. Compounds 6b′ and 6g′ demonstrated promising results against all the tested cell lines. Further, Microscopic view clearly indicated formation of apoptotic bodies, chromatin condensation, shrinkage of cells and bleb formation. Validation of the results was achieved using molecular docking studies. From the obtained results, it was observed that cyclization (oxadiazole) favored antimalarial activity while non-cyclized compounds (amides) emerged as better anticancer agents.
中文翻译:

吡唑丙烯酸基恶二唑和酰胺衍生物的合成作为抗疟和抗癌剂
就人类的发病率和死亡率而言,疟疾的恶化使它成为全球热带和亚热带地区的主要健康问题。药品伪造和不遵守治疗方案极大地促进了多药耐药性的发展和传播,这突出了开发新型和更有效的抗疟药的必要性。与癌症疾病相关的复杂性以及多种细胞群体的普遍性为癌症的治疗提供了高度特异性的治疗选择。对这些抗癌药的耐药性对成功治疗癌症构成了极大的障碍。考虑到这种持续发展的状况,人们推测它可以开发出针对疟疾和癌症的新型化合物。在同一个过道上6a–x; 6a'–h')。Schizont成熟抑制试验被用来确定抗疟的潜力。化合物6v成为靶向falcipain-2酶的最有效的抗疟药。使用磺基罗丹明B测定法进行抗癌活性。化合物6b'和6g'针对所有测试的细胞系显示出令人鼓舞的结果。此外,显微镜观察清楚地表明了凋亡小体的形成,染色质凝结,细胞皱缩和气泡形成。使用分子对接研究对结果进行了验证。从获得的结果中,观察到环化(恶二唑)有利于抗疟活性,而非环化的化合物(酰胺)作为更好的抗癌剂出现。


























 京公网安备 11010802027423号
京公网安备 11010802027423号