当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ultrafast stimulated emission of nitrophenolates in organic and aqueous solutions†
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1039/c7cp07774b N. C. Michenfelder 1, 2, 3 , H. A. Ernst 1, 2, 3 , C. Schweigert 1, 2, 3 , M. Olzmann 1, 2, 3 , A.-N. Unterreiner 1, 2, 3
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1039/c7cp07774b N. C. Michenfelder 1, 2, 3 , H. A. Ernst 1, 2, 3 , C. Schweigert 1, 2, 3 , M. Olzmann 1, 2, 3 , A.-N. Unterreiner 1, 2, 3
Affiliation
|
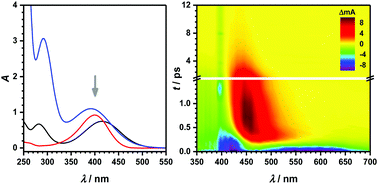
Early-time dynamics of nitroaromatics and its coressponding bases can give valuable insights into photo-induced reactions relevant to atmospheric and environmental processes. In this work, femtosecond broadband absorption spectroscopy between 350 and 700 nm has been applied to explore the ultrafast dynamics of o-, p- and m-nitrophenol anions (NP−) in basic organic and aqueous solution. Excitation at 400 nm promotes these compounds into the first bright electronic singlet state, which is a charge-transfer state. A surprising finding for all nitrophenolates was a characteristic, spectrally broad stimulated emission (SE) from the electronically excited state into the ground state. The corresponding lifetime was on the order of a few hundred femtoseconds for o- and p-NP− while it was roughly ten times larger for m-NP−. In line with earlier observations, the SE is governed by an out-of-plane torsional motion of the nitro group, leading to a close energetic approach of the relevant electronically excited singlet and ground states. Subsequent dynamics can be assigned to excited state absorption and ground state relaxation due to energy dissipation of the vibrational modes to the solvent that occur for up to several tens of picoseconds. No longer-lasting transient absorption (TA) was found; instead, a complete recovery of the ground state bleaching was observed indicating that triplet state relaxation is either not significantly involved in this spectral part or shifted to other regions. In the aqueous system, time constants for all processes are much smaller than in organic solution, a fact that can be explained by the larger dipole moment of the solvent and the correspondingly stronger intermolecular coupling between NP− and the aqueous solvent.
中文翻译:

有机和水溶液中硝基酚盐的超快激发发射†
硝基芳烃及其核心碱的早期动力学可以为与大气和环境过程有关的光致反应提供有价值的见解。在这项工作中,350和700之间处已经应用飞秒宽带吸收光谱探索的超快动力学ø - ,p -和米硝基苯酚阴离子(NP -)在碱性有机和水溶液中。在400 nm处激发会促使这些化合物进入第一个明亮的电子单重态,这是一种电荷转移态。所有硝基酚盐的一个令人惊讶的发现是从电子激发态到基态的特征性,光谱宽的受激发射(SE)。相应的寿命为几百飞秒为量级ø -和p -NP -而这是对于较大的大约10倍米-NP -。与早期的观察结果一致,SE受硝基基团的平面外扭转运动支配,从而导致了相关的电子激发单重态和基态的紧密能量途径。后续的动力学可以归因于振动模式对溶剂的能量耗散,而这种耗散会持续数十皮秒,从而激发态吸收和基态弛豫。找不到持久的瞬态吸收(TA);取而代之的是,观察到基态漂白的完全恢复,表明三重态弛豫不明显参与该光谱部分或转移到其他区域。在水性体系中,所有过程的时间常数都比有机溶液中的时间常数小得多,-和水性溶剂。
更新日期:2018-01-10
中文翻译:

有机和水溶液中硝基酚盐的超快激发发射†
硝基芳烃及其核心碱的早期动力学可以为与大气和环境过程有关的光致反应提供有价值的见解。在这项工作中,350和700之间处已经应用飞秒宽带吸收光谱探索的超快动力学ø - ,p -和米硝基苯酚阴离子(NP -)在碱性有机和水溶液中。在400 nm处激发会促使这些化合物进入第一个明亮的电子单重态,这是一种电荷转移态。所有硝基酚盐的一个令人惊讶的发现是从电子激发态到基态的特征性,光谱宽的受激发射(SE)。相应的寿命为几百飞秒为量级ø -和p -NP -而这是对于较大的大约10倍米-NP -。与早期的观察结果一致,SE受硝基基团的平面外扭转运动支配,从而导致了相关的电子激发单重态和基态的紧密能量途径。后续的动力学可以归因于振动模式对溶剂的能量耗散,而这种耗散会持续数十皮秒,从而激发态吸收和基态弛豫。找不到持久的瞬态吸收(TA);取而代之的是,观察到基态漂白的完全恢复,表明三重态弛豫不明显参与该光谱部分或转移到其他区域。在水性体系中,所有过程的时间常数都比有机溶液中的时间常数小得多,-和水性溶剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号