当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Thelepamide via Catalyst-Controlled 1,4-Addition of Cysteine Derivatives and Structure Revision of Thelepamide
Organic Letters ( IF 5.2 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.orglett.7b03706 Tobias Seitz 1 , Ramón E. Millán 2 , Dieter Lentz 1 , Carlos Jiménez 2 , Jaime Rodríguez 2 , Mathias Christmann 1
Organic Letters ( IF 5.2 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.orglett.7b03706 Tobias Seitz 1 , Ramón E. Millán 2 , Dieter Lentz 1 , Carlos Jiménez 2 , Jaime Rodríguez 2 , Mathias Christmann 1
Affiliation
|
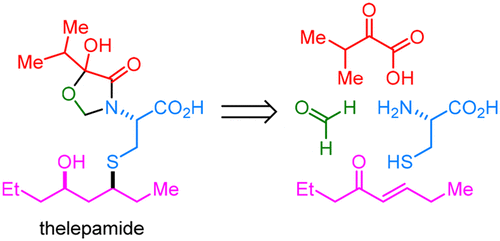
The first enantioselective total synthesis and structural reassignment of (−)-thelepamide, a cytotoxic tetraketide–amino acid from the marine worm Thelepus crispus, is reported. A convergent approach provides access to all thelepamide diastereomers in six steps from four simple building blocks. Key features of the synthesis include the application of Melchiorre’s organocatalytic thia-Michael reaction and a sonication-assisted assembly of an unprecedented N,O-acetal–hemiacetal moiety. The corrected structure was confirmed by NMR–DFT analysis.
中文翻译:

通过催化剂控制的半胱氨酸衍生物的1,4-加成合成Thelepamide和Thelepamide的结构修改
据报道,首次从海洋蠕虫Thelepus crispus获得了具有细胞毒性的四酮化合物-氨基酸-(-)-thelepamide的对映选择性全合成和结构重新分配。收敛方法可从四个简单的构建基元中以六个步骤访问所有的lepamide非对映异构体。合成的关键特征包括应用Melchiorre的有机催化thia-Michael反应和超声辅助的N,O-乙缩醛-半缩醛部分的组装。通过NMR-DFT分析确认了校正后的结构。
更新日期:2018-01-10
中文翻译:

通过催化剂控制的半胱氨酸衍生物的1,4-加成合成Thelepamide和Thelepamide的结构修改
据报道,首次从海洋蠕虫Thelepus crispus获得了具有细胞毒性的四酮化合物-氨基酸-(-)-thelepamide的对映选择性全合成和结构重新分配。收敛方法可从四个简单的构建基元中以六个步骤访问所有的lepamide非对映异构体。合成的关键特征包括应用Melchiorre的有机催化thia-Michael反应和超声辅助的N,O-乙缩醛-半缩醛部分的组装。通过NMR-DFT分析确认了校正后的结构。


























 京公网安备 11010802027423号
京公网安备 11010802027423号