当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
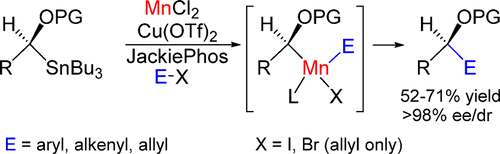
Stereospecific Stille Cross-Couplings Using Mn(II)Cl2.
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.joc.7b02780 Rambabu Dakarapu 1 , John R Falck 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.joc.7b02780 Rambabu Dakarapu 1 , John R Falck 1
Affiliation
|
Cross-coupling reactions are a staple in organic synthesis, especially for C–C bond formation with sp- and sp2-carbon electrophiles. In recent years, the range of accessible C–C bonds has been extended to stereogenic centers which expedites access to greater molecular complexity. However, these reactions predominantly depend upon late transition metal (LTM) catalysts whose cost, toxicity, and/or environmental impact have come under increasing scrutiny and governmental regulation. Here, we report Mn(II)Cl2 complexes alone, or with assistance from copper, catalyze the stereospecific cross-coupling of α-alkoxyalkylstannanes with organic electrophiles with complete retention of configuration.
中文翻译:

使用Mn(II)Cl2的立体定向Stille交叉偶联。
交叉偶联反应是有机合成中的重要部分,特别是对于使用sp-和sp 2-碳亲电试剂形成C–C键的情况。近年来,可及的C–C键的范围已扩展至立体异构中心,从而加快了获得更大分子复杂性的机会。但是,这些反应主要取决于后期过渡金属(LTM)催化剂,其成本,毒性和/或环境影响已受到越来越多的审查和政府监管。在这里,我们报告Mn(II)Cl 2配合物单独或在铜的协助下,催化α-烷氧基烷基锡烷与有机亲电体的立体定向交叉偶联,并完全保留构型。
更新日期:2018-01-17
中文翻译:

使用Mn(II)Cl2的立体定向Stille交叉偶联。
交叉偶联反应是有机合成中的重要部分,特别是对于使用sp-和sp 2-碳亲电试剂形成C–C键的情况。近年来,可及的C–C键的范围已扩展至立体异构中心,从而加快了获得更大分子复杂性的机会。但是,这些反应主要取决于后期过渡金属(LTM)催化剂,其成本,毒性和/或环境影响已受到越来越多的审查和政府监管。在这里,我们报告Mn(II)Cl 2配合物单独或在铜的协助下,催化α-烷氧基烷基锡烷与有机亲电体的立体定向交叉偶联,并完全保留构型。



























 京公网安备 11010802027423号
京公网安备 11010802027423号