当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Spectroscopic Study on the Nitrogen Electrochemical Reduction Reaction on Gold and Platinum Surfaces
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-01-18 , DOI: 10.1021/jacs.7b12101 Yao Yao 1, 2 , Shangqian Zhu 1, 2 , Haijiang Wang 1, 2 , Hui Li 1, 2 , Minhua Shao 1, 2
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-01-18 , DOI: 10.1021/jacs.7b12101 Yao Yao 1, 2 , Shangqian Zhu 1, 2 , Haijiang Wang 1, 2 , Hui Li 1, 2 , Minhua Shao 1, 2
Affiliation
|
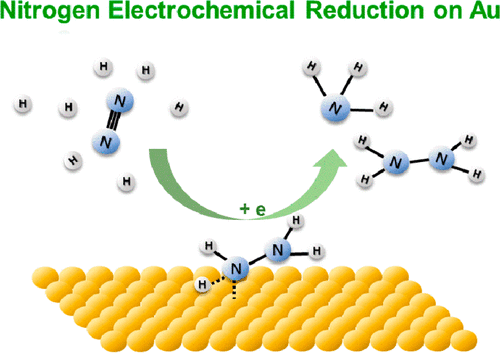
The electrochemical reduction of nitrogen to ammonia on Au-based catalysts showed a reasonably high Coulombic efficiency. The pathway of this promising reaction, however, is not clear partially due to the lack of information on reaction intermediates. Herein, surface-enhanced infrared absorption spectroscopy (SEIRAS) was employed to study the reaction mechanisms of nitrogen reduction on an Au thin film for the first time. During the nitrogen reduction, the N2Hy species was detected with bands at 1453 (H-N-H bending), 1298 (-NH2 wagging), and 1109 cm-1 (N-N stretching) at potentials below 0 V against reversible hydrogen electrode. This result indicates that the nitrogen reduction reaction on Au surfaces follows an associative mechanism, and the N≡N bond in N2 tends to break simultaneously with the hydrogen addition. By comparison, no absorption band associated with N was observed on Pt surfaces under the same reaction condition. This result is consistent with the low efficiency of nitrogen reduction on Pt due to the much faster kinetics of hydrogen evolution reaction.
中文翻译:

金和铂表面氮电化学还原反应的光谱研究
在 Au 基催化剂上将氮电化学还原为氨显示出相当高的库仑效率。然而,由于缺乏关于反应中间体的信息,这一有希望的反应的途径尚不清楚。在此,首次采用表面增强红外吸收光谱(SEIRAS)研究了金薄膜上氮还原的反应机理。在氮还原过程中,N2Hy 物质在 1453(HNH 弯曲)、1298(-NH2 摆动)和 1109 cm-1(NN 拉伸)处检测到带,电位低于 0 V,与可逆氢电极相对。该结果表明,Au 表面的氮还原反应遵循缔合机制,N2 中的 N≡N 键往往与氢的加入同时断裂。通过对比,在相同的反应条件下,在 Pt 表面没有观察到与 N 相关的吸收带。由于析氢反应的动力学快得多,该结果与 Pt 上氮还原的低效率一致。
更新日期:2018-01-18
中文翻译:

金和铂表面氮电化学还原反应的光谱研究
在 Au 基催化剂上将氮电化学还原为氨显示出相当高的库仑效率。然而,由于缺乏关于反应中间体的信息,这一有希望的反应的途径尚不清楚。在此,首次采用表面增强红外吸收光谱(SEIRAS)研究了金薄膜上氮还原的反应机理。在氮还原过程中,N2Hy 物质在 1453(HNH 弯曲)、1298(-NH2 摆动)和 1109 cm-1(NN 拉伸)处检测到带,电位低于 0 V,与可逆氢电极相对。该结果表明,Au 表面的氮还原反应遵循缔合机制,N2 中的 N≡N 键往往与氢的加入同时断裂。通过对比,在相同的反应条件下,在 Pt 表面没有观察到与 N 相关的吸收带。由于析氢反应的动力学快得多,该结果与 Pt 上氮还原的低效率一致。



























 京公网安备 11010802027423号
京公网安备 11010802027423号