当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reaction Intermediates of Nitric Oxide Synthase from Deinococcus radiodurans as Revealed by Pulse Radiolysis: Evidence for Intramolecular Electron Transfer from Biopterin to FeII–O2 Complex
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.biochem.7b00887 Yuko Tsutsui 1 , Kazuo Kobayashi 1 , Fusako Takeuchi 2 , Motonari Tsubaki 3 , Takahiro Kozawa 1
Biochemistry ( IF 2.9 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.biochem.7b00887 Yuko Tsutsui 1 , Kazuo Kobayashi 1 , Fusako Takeuchi 2 , Motonari Tsubaki 3 , Takahiro Kozawa 1
Affiliation
|
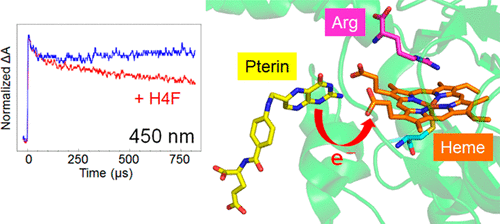
Nitric oxide synthase (NOS) is a cytochrome P450-type mono-oxygenase that catalyzes the oxidation of l-arginine (Arg) to nitric oxide (NO) through a reaction intermediate N-hydroxy-l-arginine (NHA). The mechanism underlying the reaction catalyzed by NOS from Deinococcus radiodurans was investigated using pulse radiolysis. Radiolytically generated hydrated electrons reduced the heme iron of NOS within 2 μs. Subsequently, ferrous heme reacted with O2 to form a ferrous-dioxygen intermediate with a second-order rate constant of 2.8 × 108 M–1 s–1. In the tetrahydrofolate (H4F)-bound enzyme, the ferrous-dioxygen intermediate was found to decay an another intermediate with a first-order rate constant of 2.2 × 103 s–1. The spectrum of the intermediate featured an absorption maximum at 440 nm and an absorption minimum at 390 nm. In the absence of H4F, this step did not proceed, suggesting that H4F was reduced with the ferrous-dioxygen intermediate to form a second intermediate. The intermediate further converted to the original ferric form with a first-order rate constant of 4 s–1. A similar intermediate could be detected after pulse radiolysis in the presence of NHA, although the intermediate decayed more slowly (0.5 s–1). These data suggested that a common catalytically active intermediate involved in the substrate oxidation of both Arg and NHA may be formed during catalysis. In addition, we investigated the solvent isotope effects on the kinetics of the intermediate after pulse radiolysis. Our experiments revealed dramatic kinetic solvent isotope effects on the conversion of the intermediate to the ferric form, of 10.5 and 2.5 for Arg and NHA, respectively, whereas the faster phases were not affected. These data suggest that the proton transfer in DrNOS is the rate-limiting reaction of the intermediate with the substrates.
中文翻译:

脉冲放射分解揭示了来自放射性球菌的一氧化氮合酶的反应中间体:分子内电子从生物蝶呤转移到Fe II -O 2络合物的证据
一氧化氮合酶(NOS)是一种细胞色素P450型单加氧酶,可通过反应中间体N-羟基-1-精氨酸(NHA)催化1-精氨酸(Arg)氧化为一氧化氮(NO )。利用脉冲放射分解研究了由放射线虫(Deinococcus radiodurans)的NOS催化的反应的潜在机理。辐射产生的水合电子在2μs内还原了NOS的血红素铁。随后,亚铁血红素与O 2反应形成二阶速率常数为2.8×10 8 M –1 s –1的亚铁二氧中间体。在四氢叶酸(H 4F)结合酶,发现亚铁-二氧中间体会降解另一种中间体,其一级速率常数为2.2×10 3 s –1。中间体的光谱具有在440 nm处的最大吸收和在390 nm处的最小吸收的特征。在不存在H 4 F的情况下,该步骤没有进行,表明H 4 F被亚铁-二氧中间体还原而形成第二中间体。中间体进一步转化为原始铁的形式,其一级速率常数为4 s –1。在存在NHA的情况下进行脉冲辐射分解后,可以检测到类似的中间体,尽管该中间体的降解速度较慢(0.5 s –1)。这些数据表明,在催化过程中可能形成参与Arg和NHA的底物氧化的常见催化活性中间体。另外,我们研究了脉冲辐解后溶剂同位素对中间体动力学的影响。我们的实验表明,对于中间体到三价铁的转化,动力学溶剂同位素具有显着的动力学影响,分别为Arg和NHA,分别为10.5和2.5,而更快的相不受影响。这些数据表明,DrNOS中的质子转移是中间体与底物的限速反应。
更新日期:2018-01-10
中文翻译:

脉冲放射分解揭示了来自放射性球菌的一氧化氮合酶的反应中间体:分子内电子从生物蝶呤转移到Fe II -O 2络合物的证据
一氧化氮合酶(NOS)是一种细胞色素P450型单加氧酶,可通过反应中间体N-羟基-1-精氨酸(NHA)催化1-精氨酸(Arg)氧化为一氧化氮(NO )。利用脉冲放射分解研究了由放射线虫(Deinococcus radiodurans)的NOS催化的反应的潜在机理。辐射产生的水合电子在2μs内还原了NOS的血红素铁。随后,亚铁血红素与O 2反应形成二阶速率常数为2.8×10 8 M –1 s –1的亚铁二氧中间体。在四氢叶酸(H 4F)结合酶,发现亚铁-二氧中间体会降解另一种中间体,其一级速率常数为2.2×10 3 s –1。中间体的光谱具有在440 nm处的最大吸收和在390 nm处的最小吸收的特征。在不存在H 4 F的情况下,该步骤没有进行,表明H 4 F被亚铁-二氧中间体还原而形成第二中间体。中间体进一步转化为原始铁的形式,其一级速率常数为4 s –1。在存在NHA的情况下进行脉冲辐射分解后,可以检测到类似的中间体,尽管该中间体的降解速度较慢(0.5 s –1)。这些数据表明,在催化过程中可能形成参与Arg和NHA的底物氧化的常见催化活性中间体。另外,我们研究了脉冲辐解后溶剂同位素对中间体动力学的影响。我们的实验表明,对于中间体到三价铁的转化,动力学溶剂同位素具有显着的动力学影响,分别为Arg和NHA,分别为10.5和2.5,而更快的相不受影响。这些数据表明,DrNOS中的质子转移是中间体与底物的限速反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号