当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Conformational stabilization of a β-hairpin through a triazole–tryptophan interaction†
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1039/c7ob02815f Donatella Diana 1, 2, 3, 4 , Claudia Di Salvo 1, 2, 3, 4 , Veronica Celentano 1, 2, 3, 4 , Lucia De Rosa 1, 2, 3, 4 , Alessandra Romanelli 3, 4, 5, 6 , Roberto Fattorusso 4, 7, 8, 9, 10 , Luca D. D'Andrea 1, 2, 3, 4
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1039/c7ob02815f Donatella Diana 1, 2, 3, 4 , Claudia Di Salvo 1, 2, 3, 4 , Veronica Celentano 1, 2, 3, 4 , Lucia De Rosa 1, 2, 3, 4 , Alessandra Romanelli 3, 4, 5, 6 , Roberto Fattorusso 4, 7, 8, 9, 10 , Luca D. D'Andrea 1, 2, 3, 4
Affiliation
|
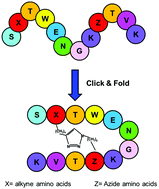
Molecular tools to stabilize the β-hairpin conformation are needed as β-hairpin peptides are useful molecules for pharmaceutical, biological and materials applications. We explored the use of a “triazole bridge”, a covalent link between two β-hairpin strands obtained through Cu-catalyzed alkyne–azide cycloaddition, combined with an aromatic–aromatic interaction. Highly conformationally stable peptides were identified by NMR screening of a small collection of cyclic peptides based on the Trpzip2 scaffold. The characteristic Trp–Trp interaction of Trpzip2 was replaced by a diagonal triazole bridge of variable length. NMR and CD analyses showed that triazole and indole rings could favorably interact to stabilize a β-hairpin conformation. The conformational stabilization depends on the length of the triazole bridge and the reciprocal position between the aromatic rings. Combining aromatic interactions and the covalent inter-strand triazole bridge is a useful strategy to obtain peptides with a high β-hairpin content.
中文翻译:

通过三唑-色氨酸相互作用构筑β-发夹的构象稳定化†
需要稳定β-发夹结构的分子工具,因为β-发夹肽是用于药物,生物学和材料应用的有用分子。我们探索了“三唑桥”的使用,该三唑桥是通过Cu催化的炔-叠氮化物环加成反应与芳香族-芳族相互作用结合而获得的两个β-发夹链之间的共价连接。通过NMR筛选基于Trpzip2支架的少量环状肽,可以鉴定出高度构象稳定的肽。Trpzip2的特征性Trp-Trp相互作用被可变长度的对角三唑桥所取代。NMR和CD分析表明,三唑和吲哚环可以有利地相互作用以稳定β-发夹构象。构象稳定取决于三唑桥的长度和芳环之间的相对位置。结合芳族相互作用和共价链间三唑桥是获得具有高β-发夹含量的肽的有用策略。
更新日期:2018-01-10
中文翻译:

通过三唑-色氨酸相互作用构筑β-发夹的构象稳定化†
需要稳定β-发夹结构的分子工具,因为β-发夹肽是用于药物,生物学和材料应用的有用分子。我们探索了“三唑桥”的使用,该三唑桥是通过Cu催化的炔-叠氮化物环加成反应与芳香族-芳族相互作用结合而获得的两个β-发夹链之间的共价连接。通过NMR筛选基于Trpzip2支架的少量环状肽,可以鉴定出高度构象稳定的肽。Trpzip2的特征性Trp-Trp相互作用被可变长度的对角三唑桥所取代。NMR和CD分析表明,三唑和吲哚环可以有利地相互作用以稳定β-发夹构象。构象稳定取决于三唑桥的长度和芳环之间的相对位置。结合芳族相互作用和共价链间三唑桥是获得具有高β-发夹含量的肽的有用策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号