当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrostatic Interactions in Protein Structure, Folding, Binding, and Condensation
Chemical Reviews ( IF 62.1 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.chemrev.7b00305 Huan-Xiang Zhou 1, 2 , Xiaodong Pang 2
Chemical Reviews ( IF 62.1 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acs.chemrev.7b00305 Huan-Xiang Zhou 1, 2 , Xiaodong Pang 2
Affiliation
|
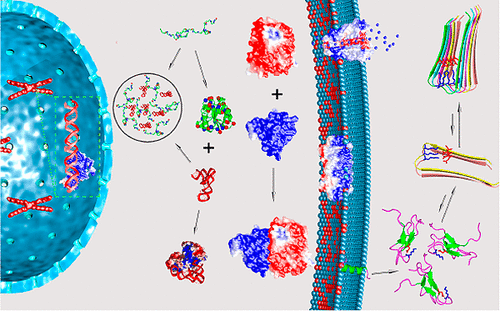
Charged and polar groups, through forming ion pairs, hydrogen bonds, and other less specific electrostatic interactions, impart important properties to proteins. Modulation of the charges on the amino acids, e.g., by pH and by phosphorylation and dephosphorylation, have significant effects such as protein denaturation and switch-like response of signal transduction networks. This review aims to present a unifying theme among the various effects of protein charges and polar groups. Simple models will be used to illustrate basic ideas about electrostatic interactions in proteins, and these ideas in turn will be used to elucidate the roles of electrostatic interactions in protein structure, folding, binding, condensation, and related biological functions. In particular, we will examine how charged side chains are spatially distributed in various types of proteins and how electrostatic interactions affect thermodynamic and kinetic properties of proteins. Our hope is to capture both important historical developments and recent experimental and theoretical advances in quantifying electrostatic contributions of proteins.
中文翻译:

蛋白质结构,折叠,结合和缩合中的静电相互作用
带电和极性基团通过形成离子对,氢键和其他较不特异的静电相互作用,为蛋白质赋予重要的性能。氨基酸上电荷的调节,例如通过pH调节以及通过磷酸化和去磷酸化,具有重要的作用,例如蛋白质变性和信号转导网络的开关样响应。这篇综述旨在提出蛋白质电荷和极性基团的各种作用之间的统一主题。简单的模型将用于说明有关蛋白质中静电相互作用的基本概念,而这些思想又将用于阐明静电相互作用在蛋白质结构,折叠,结合,缩合和相关生物学功能中的作用。尤其是,我们将研究带电侧链如何在空间上分布在各种类型的蛋白质中,以及静电相互作用如何影响蛋白质的热力学和动力学性质。我们的希望是捕捉重要的历史发展以及量化蛋白质的静电作用的最新实验和理论进展。
更新日期:2018-01-10
中文翻译:

蛋白质结构,折叠,结合和缩合中的静电相互作用
带电和极性基团通过形成离子对,氢键和其他较不特异的静电相互作用,为蛋白质赋予重要的性能。氨基酸上电荷的调节,例如通过pH调节以及通过磷酸化和去磷酸化,具有重要的作用,例如蛋白质变性和信号转导网络的开关样响应。这篇综述旨在提出蛋白质电荷和极性基团的各种作用之间的统一主题。简单的模型将用于说明有关蛋白质中静电相互作用的基本概念,而这些思想又将用于阐明静电相互作用在蛋白质结构,折叠,结合,缩合和相关生物学功能中的作用。尤其是,我们将研究带电侧链如何在空间上分布在各种类型的蛋白质中,以及静电相互作用如何影响蛋白质的热力学和动力学性质。我们的希望是捕捉重要的历史发展以及量化蛋白质的静电作用的最新实验和理论进展。



























 京公网安备 11010802027423号
京公网安备 11010802027423号