当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sustainable and Efficient CuI‐NPs‐Catalyzed Cross‐Coupling Approach for the Synthesis of Tertiary 3‐Aminopropenoates, Triazoles, and Ciprofloxacin
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2018-02-01 , DOI: 10.1002/ajoc.201700682 Onkar S. Nayal 1, 2 , Maheshwar S. Thakur 1, 2 , Manoranjan Kumar 1, 2 , Shaifali 1, 2 , Rahul Upadhyay 1 , Sushil K. Maurya 1, 2
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2018-02-01 , DOI: 10.1002/ajoc.201700682 Onkar S. Nayal 1, 2 , Maheshwar S. Thakur 1, 2 , Manoranjan Kumar 1, 2 , Shaifali 1, 2 , Rahul Upadhyay 1 , Sushil K. Maurya 1, 2
Affiliation
|
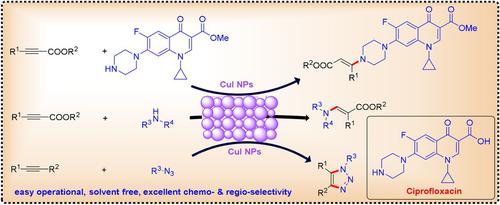
The unique properties of metal nanoparticles have attracted significant attention from synthetic chemists, notably for application in heterogeneous catalysis. Herein, we report a highly efficient, sustainable, and atom‐economical approach for the synthesis of tertiary 3‐aminopropenoates from alkynes and secondary amines by using heterogeneous copper(I) iodide nanoparticles (CuI NPs) as a catalyst. The broad utility of this strategy is demonstrated by the synthesis of a wide range of tertiary 3‐aminopropenoates. The value of this transformation has been highlighted by the synthesis of biologically active ciprofloxacin and its derivatives on a gram scale. This method was also applied to the synthesis of 1,4‐disubstituted 1,2,3‐triazoles.
中文翻译:

可持续和高效的CuI-NPs催化交叉偶联方法合成3-氨基氨基丙酸酯,三唑和环丙沙星
金属纳米颗粒的独特性能引起了合成化学家的极大关注,特别是在多相催化中的应用。本文中,我们报告了一种高效,可持续和原子经济的方法,可通过使用异质碘化铜(I)纳米粒子(CuI NPs)作为催化剂,从炔烃和仲胺合成叔3-氨基丙酸酯。广泛的3-氨基丙酸酯叔胺的合成证明了该策略的广泛用途。通过克量级的生物活性环丙沙星及其衍生物的合成突出了这种转化的价值。该方法也适用于1,4-二取代的1,2,3-三唑的合成。
更新日期:2018-02-01
中文翻译:

可持续和高效的CuI-NPs催化交叉偶联方法合成3-氨基氨基丙酸酯,三唑和环丙沙星
金属纳米颗粒的独特性能引起了合成化学家的极大关注,特别是在多相催化中的应用。本文中,我们报告了一种高效,可持续和原子经济的方法,可通过使用异质碘化铜(I)纳米粒子(CuI NPs)作为催化剂,从炔烃和仲胺合成叔3-氨基丙酸酯。广泛的3-氨基丙酸酯叔胺的合成证明了该策略的广泛用途。通过克量级的生物活性环丙沙星及其衍生物的合成突出了这种转化的价值。该方法也适用于1,4-二取代的1,2,3-三唑的合成。


























 京公网安备 11010802027423号
京公网安备 11010802027423号