当前位置:
X-MOL 学术
›
Electroanalysis
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Studies of Electrode Reactions and Coordination Geometries of Cu(I) and Cu(II) Complexes with Bicinchoninic Acid
Electroanalysis ( IF 3 ) Pub Date : 2018-01-10 , DOI: 10.1002/elan.201700741 Dinglong Chen 1 , Xiaoying Wang 1 , Yonghui He 2 , Tianhan Kai 3 , Zhiqiang Li 3 , Juan Xiang 1 , Dianlu Jiang 3 , Feimeng Zhou 3
Electroanalysis ( IF 3 ) Pub Date : 2018-01-10 , DOI: 10.1002/elan.201700741 Dinglong Chen 1 , Xiaoying Wang 1 , Yonghui He 2 , Tianhan Kai 3 , Zhiqiang Li 3 , Juan Xiang 1 , Dianlu Jiang 3 , Feimeng Zhou 3
Affiliation
|
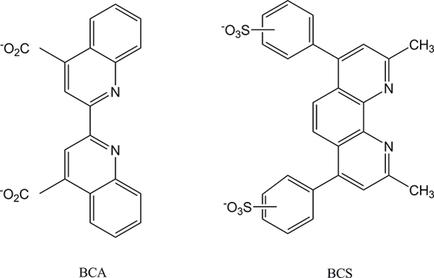
Bicinchoninic acid (BCA) is widely used for determining the valence state of copper in biological systems and quantification of the total protein concentration (BCA assay). Despite its well‐known high selectivity of Cu(I) over Cu(II), the exact formation constants for Cu(I)(BCA)23− and Cu(II)(BCA)22− complexes remain uncertain. These uncertainties, affect the correct interpretations of the roles of copper in biological processes and the BCA assay data. By studying the voltammetric behaviors of Cu(I)(BCA)23− and Cu(II)(BCA)22−, we demonstrate that the apparent lack of redox reaction reversibility is caused by an adsorption wave of Cu(II)(BCA)22−. With the adsorption wave identified, we found that the Cu(I)/Cu(II) selectivity of BCA is essentially identical to another popular ligand, bathocuproinedisulfonic acid (BCS). Density functional theory calculation on the geometries of Cu(I)(BCA)23− and Cu(II)(BCA)22− rationalizes the preferential Cu(I) binding by BCA and the strong adsorption of the Cu(II)(BCA)22− complex at the glassy carbon electrode. Based on the shift in the standard reduction potential of free Cu(II)/Cu(I) upon binding to BCA, we affirm that the formation constants for Cu(I)(BCA)23− and Cu(II)(BCA)22− are 1017.2 and 108.9, respectively. Therefore, BCA can be chosen among various ligands for effective and reliable studies of the copper binding affinities of different biomolecules.
中文翻译:

Cu(I)和Cu(II)与双辛可宁酸配合物的电极反应和配位几何结构的研究
双辛可宁酸(BCA)被广泛用于确定生物系统中铜的价态和定量总蛋白浓度(BCA分析)。尽管Cu(I)对Cu(II)具有很高的选择性,但是Cu(I)(BCA)2 3-和Cu(II)(BCA)2 2-配合物的确切形成常数仍然不确定。这些不确定性影响对铜在生物过程中的作用和BCA分析数据的正确解释。通过研究Cu(I)(BCA)2 3-和Cu(II)(BCA)2 2-的伏安行为,我们证明了氧化还原反应可逆性的明显缺乏是由于Cu(II)( BCA)2 2−。识别出吸附波后,我们发现BCA的Cu(I)/ Cu(II)选择性与另一种流行的配体,浴铜素二磺酸(BCS)基本相同。Cu(I)(BCA)2 3−和Cu(II)(BCA)2 2−的几何结构的密度泛函理论计算合理化了BCA优先的Cu(I)结合和Cu(II)( BCA)2 2-在玻璃碳电极上的络合物。基于游离Cu(II)/ Cu(I)与BCA结合后标准还原电位的变化,我们确认Cu(I)(BCA)2 3-和Cu(II)(BCA)的形成常数2 2−是10 17.2和10 8.9, 分别。因此,可以在各种配体中选择BCA,以有效而可靠地研究不同生物分子的铜结合亲和力。
更新日期:2018-01-10
中文翻译:

Cu(I)和Cu(II)与双辛可宁酸配合物的电极反应和配位几何结构的研究
双辛可宁酸(BCA)被广泛用于确定生物系统中铜的价态和定量总蛋白浓度(BCA分析)。尽管Cu(I)对Cu(II)具有很高的选择性,但是Cu(I)(BCA)2 3-和Cu(II)(BCA)2 2-配合物的确切形成常数仍然不确定。这些不确定性影响对铜在生物过程中的作用和BCA分析数据的正确解释。通过研究Cu(I)(BCA)2 3-和Cu(II)(BCA)2 2-的伏安行为,我们证明了氧化还原反应可逆性的明显缺乏是由于Cu(II)( BCA)2 2−。识别出吸附波后,我们发现BCA的Cu(I)/ Cu(II)选择性与另一种流行的配体,浴铜素二磺酸(BCS)基本相同。Cu(I)(BCA)2 3−和Cu(II)(BCA)2 2−的几何结构的密度泛函理论计算合理化了BCA优先的Cu(I)结合和Cu(II)( BCA)2 2-在玻璃碳电极上的络合物。基于游离Cu(II)/ Cu(I)与BCA结合后标准还原电位的变化,我们确认Cu(I)(BCA)2 3-和Cu(II)(BCA)的形成常数2 2−是10 17.2和10 8.9, 分别。因此,可以在各种配体中选择BCA,以有效而可靠地研究不同生物分子的铜结合亲和力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号