Journal of Molecular Liquids ( IF 6 ) Pub Date : 2018-01-09 , DOI: 10.1016/j.molliq.2018.01.036 Ahmed E. Fazary , Mutasem Z. Bani-Fwaz , Khaled F. Fawy , Hisham S.M. Abd-Rabboh
|
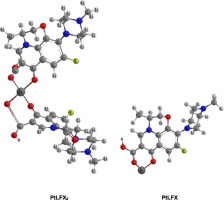
The experimental protonation and complex formation equilibrium constants of levaquin (LFX) with platinum(II) and palladium(II) metal ions have been investigated at 310.15 K in water solutions at ionic strength of I = 0.16 mol·dm− 3 NaNO3 using pH-potentiometric and cyclic voltammetry techniques, and by means of Hyperquad 2008 estimation model program. Also, the dissociation constants of LFX and the equilibrium constants of its binary complexes with the studied platinum(II) and palladium(II) metal ions in I = 0.16 mol·dm− 3 NaNO3 water solutions were observed at different temperatures such as 298.15, 310.15, 318.15 and 328.15 K. The theoretical calculations of overall protonation and stability constants of the metal ion-LFX complex species in aqueous solutions were predicted as the free energy change associated with the LFX protonation, and metal ion – LFX complex formation equilibria using ab initio and density function theory calculations by applying Gaussian 09 software molecular modeling. The determination of equilibrium constants were confirmed by the spectrophotometric method with the refinement by using the HypSpec program. The usage of the experimental potentiometry/spectrophotometry techniques and theoretical predictions provides a complete picture of the microscopic equilibria of the studied systems (metal ions -LFX). Precisely, this theoretically predications could be useful to control the most real protonation constants of levaquin ligand in which the binding sites changes due to the ligand protonation/deprotonation equilibria. Also, the complexing capacities of different platinum(II) and palladium(II) metal ions towards LFX in solutions were evaluated and discussed. From the determined experimental stability constants of different metal complex species, the concentration distribution diagrams of the various metal ions - LFX complex species in solutions were estimated using HySS 2009 software.
中文翻译:

Levaquin药物对铂和钯金属离子的络合特性:水溶液中的热力学研究
在pH值为I = 0.16 mol·dm − 3 NaNO 3的水溶液中,在310.15 K的条件下研究了左旋喹啉与铂(II)和钯(II)金属离子的质子化和络合物形成平衡常数。-电位和循环伏安法技术,并通过Hyperquad 2008估算模型程序进行。此外,在I = 0.16 mol·dm -3 NaNO 3中,LFX的解离常数及其与所研究的铂(II)和钯(II)金属离子的二元配合物的平衡常数。在298.15、310.15、318.15和328.15 K等不同温度下观察到水溶液。金属离子-LFX配合物物种在水溶液中的整体质子化和稳定性常数的理论计算被预测为与LFX质子化相关的自由能变化,以及通过应用Gaussian 09软件分子建模,使用从头算和密度泛函理论计算的金属离子-LFX络合物形成平衡。平衡常数的测定通过分光光度法通过使用HypSpec程序进行了改进。实验电位计/分光光度法技术和理论预测的使用提供了所研究系统(金属离子-LFX)的微观平衡的完整图片。恰恰,该理论上的预测可用于控制左旋喹配体的最真实的质子化常数,其中结合位点由于配体的质子化/去质子化平衡而改变。此外,评估并讨论了不同的铂(II)和钯(II)金属离子对LFX的络合能力。根据确定的不同金属络合物物种的实验稳定性常数,使用HySS 2009软件估算溶液中各种金属离子-LFX络合物物种的浓度分布图。评估和讨论了不同铂(II)和钯(II)金属离子对溶液中LFX的络合能力。根据确定的不同金属络合物物种的实验稳定性常数,使用HySS 2009软件估算溶液中各种金属离子-LFX络合物物种的浓度分布图。评估和讨论了不同铂(II)和钯(II)金属离子对溶液中LFX的络合能力。根据确定的不同金属络合物物种的实验稳定性常数,使用HySS 2009软件估算溶液中各种金属离子-LFX络合物物种的浓度分布图。



























 京公网安备 11010802027423号
京公网安备 11010802027423号