Bioresource Technology ( IF 11.4 ) Pub Date : 2018-01-09 , DOI: 10.1016/j.biortech.2018.01.036 Michael Lienemann , Jörg Stefan Deutzmann , Ross Dean Milton , Merve Sahin , Alfred Michael Spormann
|
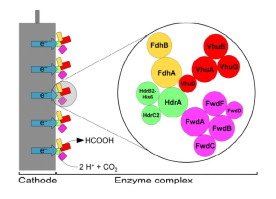
Electrosynthesis of formate is a promising technology to convert CO2 and electricity from renewable sources into a biocompatible, soluble, non-flammable, and easily storable compound. In the model methanogen Methanococcus maripaludis, uptake of cathodic electrons was shown to proceed indirectly via formation of formate or H2 by undefined, cell-derived enzymes. Here, we identified that the multi-enzyme heterodisulfide reductase supercomplex (Hdr-SC) of M. maripaludis is capable of direct electron uptake and catalyzes rapid H2 and formate formation in electrochemical reactors (−800 mV vs Ag/AgCl) and in Fe(0) corrosion assays. In Fe(0) corrosion assays and electrochemical reactors, purified Hdr-SC primarily catalyzed CO2 reduction to formate with a coulombic efficiency of 90% in the electrochemical cells for 5 days. Thus, this report identified the first enzyme that stably catalyzes the mediator-free electrochemical reduction of CO2 to formate, which can serve as the basis of an enzyme electrode for sustained electrochemical production of formate.
中文翻译:

马氏甲烷球菌杂二硫键还原酶超复合物的无介体酶促甲酸合成
甲酸的电合成是一种有前途的技术,可将来自可再生资源的CO 2和电转化为生物相容,可溶,不可燃且易于储存的化合物。在模型产甲烷菌马氏甲烷球菌中,阴极电子的摄取显示为通过不确定的细胞衍生酶形成甲酸酯或H 2间接进行。在这里,我们确定了M. maripaludis的多酶杂二硫键还原酶超复合物(Hdr-SC)能够直接吸收电子并催化快速的H 2。在电化学反应器(-800 mV,相对于Ag / AgCl)和Fe(0)腐蚀分析中形成甲酸盐。在Fe(0)腐蚀分析和电化学反应器中,纯化的Hdr-SC主要催化CO 2还原生成甲酸,在电化学电池中连续90天的库仑效率为90%。因此,本报告确定了第一种稳定催化无CO 2的电化学还原反应生成甲酸的酶,该酶可作为持续电化学生成甲酸的酶电极的基础。



























 京公网安备 11010802027423号
京公网安备 11010802027423号