Acta Biomaterialia ( IF 9.7 ) Pub Date : 2018-01-10 , DOI: 10.1016/j.actbio.2018.01.002 Shikha Chawla , Sourabh Ghosh
|
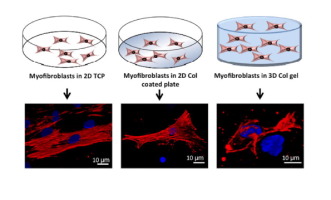
Current therapeutic strategies to reduce scarring in full thickness skin defect offer limited success due to poor understanding of scar tissue formation and the underlying signaling pathways. There is an urgent need to develop human cell based in vitro scar tissue models as animal testing is associated with ethical and logistic complications and inter-species variations. Pro-inflammatory cytokines play critical role in regulating scar development through complex interplay and interaction with the ECM and corresponding signaling pathways. In this context, we assessed the responses of cultured fibroblasts with respect to their differentiation into myofibroblasts using optimised cytokines (TGF-β1, IL-6 and IL-8) for scar formation in 2D (tissue culture plate, collagen type I coated plate) vs 3D collagen type I gel based constructs. We attempted to deduce the role of dimensionality of cell culture matrix in modulating differentiation, function and phenotype of cultured fibroblasts. Validation of the developed model showed similarity to etiology and pathophysiology of in vivo hypertrophic scar with respect to several features: 1) transition of fibroblasts to myofibroblasts with convincing expression of α-SMA stress fibers; 2) contraction; 3) excessive collagen and fibronectin secretion; 4) expression of fibrotic ECM proteins (SPARC and Tenascin); 5) low MMP secretion. Most importantly, we elucidated the involvement of TGF-β/SMAD and Wnt/β-catenin pathways in developing in vitro dermal scar. Hence, this relatively simple in vitro human scar tissue equivalent may serve as an alternative for testing and designing of novel therapeutics and help in extending our understanding of the complex interplay of cytokines and related dermal scar specific signaling.
Statement of significance
Scarring of the skin affects almost millions of people per year in the developed world alone, nevertheless the complex pathophysiology and the precise signaling mechanisms responsible for this phenomenon of skin scarring are still unknown. A number of anti-scar drugs are being developed and being tested on animals and monolayer models. However, testing the efficacy of these drugs on lab based 3D in vitro models may prove extremely useful in recapitulating the 3D microenvironment of the native scar tissue. In that context in this study we have demonstrated the development of 3D in vitro dermal scar model, by optimizing a constellation of factors, such as combination of cytokines (TGF-β1,IL-6,IL-8) and cellular dimensionality in inducing the differentiation of dermal fibroblasts to myofibroblasts. This in vitro scar model was successful in replicating hallmark features of hypertrophic scar such as excessive synthesis of fibrotic extracellular matrix, perturbed matrix homeostasis, contraction, diminished MMP synthesis. The study also highlighted significant involvement of TGF-β/SMAD and Wnt/β-catenin signaling pathways in in vitro scar formation.
中文翻译:

通过细胞因子,维数和基质的协同作用调节纤维化变化:朝着体外人类皮肤肥大性瘢痕模型的发展
由于对疤痕组织形成和潜在的信号传导途径了解不足,目前减少全层皮肤缺损疤痕的治疗策略只能提供有限的成功。迫切需要开发基于人细胞的体外动物测试中的疤痕组织模型与伦理和后勤并发症以及种间变异有关。促炎性细胞因子通过复杂的相互作用以及与ECM和相应信号通路的相互作用,在调节瘢痕形成中起关键作用。在这种情况下,我们使用优化的细胞因子(TGF-β1,IL-6和IL-8)以二维方式(组织培养板,I型胶原涂层板)形成疤痕,评估了培养的成纤维细胞分化为成肌纤维细胞的反应。 vs 3D I型胶原蛋白凝胶构建体。我们试图推断细胞培养基质的维数在调节培养成纤维细胞的分化,功能和表型中的作用。验证开发模型的相似性与病因和病理生理学相似体内肥厚性瘢痕有以下几个特征:1)令人信服的α-SMA应力纤维表达的成纤维细胞向成肌纤维细胞的转化;2)收缩;3)胶原蛋白和纤连蛋白分泌过多;4)纤维化ECM蛋白(SPARC和Tenascin)的表达;5)MMP分泌低。最重要的是,我们阐明了TGF-β/ SMAD和Wnt /β-catenin途径在体外皮肤瘢痕形成中的作用。因此,这种相对简单的体外人瘢痕组织等同物可以作为测试和设计新型疗法的替代方法,并有助于扩展我们对细胞因子和相关的皮肤瘢痕特异性信号的复杂相互作用的理解。
重要声明
仅在发达国家,皮肤的疤痕每年就影响数以百万计的人,然而,尚不清楚造成这种皮肤疤痕现象的复杂的病理生理学和精确的信号传导机制。正在开发多种抗疤痕药物,并在动物和单层模型上对其进行测试。但是,在基于实验室的3D体外模型中测试这些药物的功效可能证明在概括天然疤痕组织的3D微环境方面非常有用。在此背景下,我们证明了体外3D的发展皮肤疤痕模型,可通过优化一系列因素(例如细胞因子(TGF-β1,IL-6,IL-8)和细胞尺寸的组合)来诱导皮肤成纤维细胞向肌成纤维细胞的分化。这种体外瘢痕模型成功地复制了肥厚性瘢痕的标志性特征,例如纤维化细胞外基质的过度合成,基质稳态的扰动,收缩,MMP合成减少。该研究还强调了TGF-β/ SMAD和Wnt /β-catenin信号通路在体外瘢痕形成中的重要参与。

























 京公网安备 11010802027423号
京公网安备 11010802027423号