当前位置:
X-MOL 学术
›
ACS Synth. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reprogramming One-Carbon Metabolic Pathways To Decouple l-Serine Catabolism from Cell Growth in Corynebacterium glutamicum
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2018-01-19 00:00:00 , DOI: 10.1021/acssynbio.7b00373 Yun Zhang 1 , Xiuling Shang 1 , Shujuan Lai 1 , Yu Zhang 1, 2 , Qitiao Hu 1, 2 , Xin Chai 1 , Bo Wang 1, 2 , Shuwen Liu 1 , Tingyi Wen 1, 3
ACS Synthetic Biology ( IF 4.7 ) Pub Date : 2018-01-19 00:00:00 , DOI: 10.1021/acssynbio.7b00373 Yun Zhang 1 , Xiuling Shang 1 , Shujuan Lai 1 , Yu Zhang 1, 2 , Qitiao Hu 1, 2 , Xin Chai 1 , Bo Wang 1, 2 , Shuwen Liu 1 , Tingyi Wen 1, 3
Affiliation
|
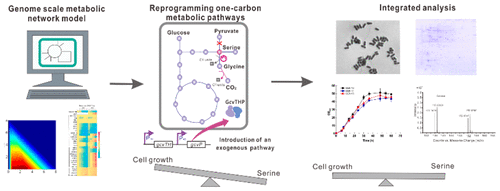
l-Serine, the principal one-carbon source for DNA biosynthesis, is difficult for microorganisms to accumulate due to the coupling of l-serine catabolism and microbial growth. Here, we reprogrammed the one-carbon unit metabolic pathways in Corynebacterium glutamicum to decouple l-serine catabolism from cell growth. In silico model-based simulation showed a negative influence on glyA-encoding serine hydroxymethyltransferase flux with l-serine productivity. Attenuation of glyA transcription resulted in increased l-serine accumulation, and a decrease in purine pools, poor growth and longer cell shapes. The gcvTHP-encoded glycine cleavage (Gcv) system from Escherichia coli was introduced into C. glutamicum, allowing glycine-derived 13CH2 to be assimilated into intracellular purine synthesis, which resulted in an increased amount of one-carbon units. Gcv introduction not only restored cell viability and morphology but also increased l-serine accumulation. Moreover, comparative proteomic analysis indicated that abundance changes of the enzymes involved in one-carbon unit cycles might be responsible for maintaining one-carbon unit homeostasis. Reprogramming of the one-carbon metabolic pathways allowed cells to reach a comparable growth rate to accumulate 13.21 g/L l-serine by fed-batch fermentation in minimal medium. This novel strategy provides new insights into the regulation of cellular properties and essential metabolite accumulation by introducing an extrinsic pathway.
中文翻译:

重编程一碳代谢途径,以从谷氨酸棒杆菌的细胞生长中分离l-丝氨酸代谢。
l-丝氨酸是DNA生物合成的主要一碳来源,由于l-丝氨酸分解代谢与微生物生长的耦合,微生物难以积聚。在这里,我们重新编程的一碳单位的代谢途径的谷氨酸棒状杆菌,以解耦升从细胞生长-丝氨酸分解代谢。在基于计算机模拟的模拟中,显示了对带有l-丝氨酸生产力的glyA编码丝氨酸羟甲基转移酶通量的负面影响。衰减glyA转录导致增加升丝氨酸积累,嘌呤池,生长不良和更长的电池形状的下降。这将来自大肠杆菌的gcvTHP编码的甘氨酸裂解(Gcv)系统引入到谷氨酸棒杆菌中,使甘氨酸衍生的13 CH 2被同化到细胞内嘌呤合成中,从而导致一碳单元的数量增加。Gcv的引入不仅恢复了细胞的活力和形态,而且还增加了1-丝氨酸的积累。此外,比较蛋白质组学分析表明,参与一碳单位循环的酶的丰度变化可能是维持一碳单位稳态的原因。重编程一碳代谢途径可使细胞达到可比的生长速度,从而积累13.21 g / L l在基本培养基中分批补料发酵生产丝氨酸。通过引入外在途径,这种新颖的策略为调节细胞特性和必需代谢产物积累提供了新的见识。
更新日期:2018-01-19
中文翻译:

重编程一碳代谢途径,以从谷氨酸棒杆菌的细胞生长中分离l-丝氨酸代谢。
l-丝氨酸是DNA生物合成的主要一碳来源,由于l-丝氨酸分解代谢与微生物生长的耦合,微生物难以积聚。在这里,我们重新编程的一碳单位的代谢途径的谷氨酸棒状杆菌,以解耦升从细胞生长-丝氨酸分解代谢。在基于计算机模拟的模拟中,显示了对带有l-丝氨酸生产力的glyA编码丝氨酸羟甲基转移酶通量的负面影响。衰减glyA转录导致增加升丝氨酸积累,嘌呤池,生长不良和更长的电池形状的下降。这将来自大肠杆菌的gcvTHP编码的甘氨酸裂解(Gcv)系统引入到谷氨酸棒杆菌中,使甘氨酸衍生的13 CH 2被同化到细胞内嘌呤合成中,从而导致一碳单元的数量增加。Gcv的引入不仅恢复了细胞的活力和形态,而且还增加了1-丝氨酸的积累。此外,比较蛋白质组学分析表明,参与一碳单位循环的酶的丰度变化可能是维持一碳单位稳态的原因。重编程一碳代谢途径可使细胞达到可比的生长速度,从而积累13.21 g / L l在基本培养基中分批补料发酵生产丝氨酸。通过引入外在途径,这种新颖的策略为调节细胞特性和必需代谢产物积累提供了新的见识。



























 京公网安备 11010802027423号
京公网安备 11010802027423号