当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Novel Tetrahydroisoquinoline CXCR4 Antagonists with Rigidified Side-Chains
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00406 Edgars Jecs 1 , Eric J. Miller 1 , Robert J. Wilson 1 , Huy H. Nguyen 1 , Yesim A. Tahirovic 1 , Brook M. Katzman 1 , Valarie M. Truax 1 , Michelle B. Kim 1 , Katie M. Kuo 1 , Tao Wang 2 , Chi S. Sum 2 , Mary E. Cvijic 2 , Gretchen M. Schroeder 2 , Lawrence J. Wilson 1 , Dennis C. Liotta 1
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1021/acsmedchemlett.7b00406 Edgars Jecs 1 , Eric J. Miller 1 , Robert J. Wilson 1 , Huy H. Nguyen 1 , Yesim A. Tahirovic 1 , Brook M. Katzman 1 , Valarie M. Truax 1 , Michelle B. Kim 1 , Katie M. Kuo 1 , Tao Wang 2 , Chi S. Sum 2 , Mary E. Cvijic 2 , Gretchen M. Schroeder 2 , Lawrence J. Wilson 1 , Dennis C. Liotta 1
Affiliation
|
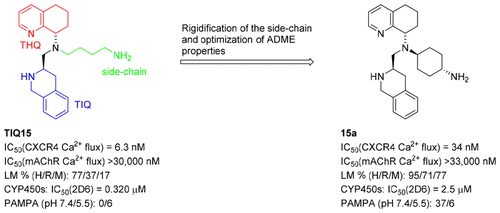
A structure–activity relationship study of potent TIQ15-derived CXCR4 antagonists is reported. In this investigation, the TIQ15 side-chain was constrained to improve its drug properties. The cyclohexylamino congener 15a was found to be a potent CXCR4 inhibitor (IC50 = 33 nM in CXCL12-mediated Ca2+ flux) with enhanced stability in liver microsomes and reduced inhibition of CYP450 (2D6). The improved CXCR4 antagonist 15a has potential therapeutic application as a single agent or combinatory anticancer therapy.
中文翻译:

具有刚性链的新型四氢异喹啉CXCR4拮抗剂的合成
报道了有效的TIQ15衍生的CXCR4拮抗剂的结构活性关系研究。在这项研究中,TIQ15侧链被限制以改善其药物特性。发现环己基氨基同源物15a是有效的CXCR4抑制剂(在CXCL12介导的Ca 2+通量中IC 50 = 33 nM ),在肝微粒体中的稳定性增强,并且对CYP450(2D6)的抑制作用降低。改进的CXCR4拮抗剂15a具有作为单药或联合抗癌疗法的潜在治疗应用。
更新日期:2018-01-09
中文翻译:

具有刚性链的新型四氢异喹啉CXCR4拮抗剂的合成
报道了有效的TIQ15衍生的CXCR4拮抗剂的结构活性关系研究。在这项研究中,TIQ15侧链被限制以改善其药物特性。发现环己基氨基同源物15a是有效的CXCR4抑制剂(在CXCL12介导的Ca 2+通量中IC 50 = 33 nM ),在肝微粒体中的稳定性增强,并且对CYP450(2D6)的抑制作用降低。改进的CXCR4拮抗剂15a具有作为单药或联合抗癌疗法的潜在治疗应用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号