当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
C8-Linked Pyrrolobenzodiazepine Monomers with Inverted Building Blocks Show Selective Activity against Multidrug Resistant Gram-Positive Bacteria
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1021/acsinfecdis.7b00130 Paolo Andriollo 1 , Charlotte K. Hind 2 , Pietro Picconi 1 , Kazi S. Nahar 1 , Shirin Jamshidi 1 , Amrit Varsha 1 , Melanie Clifford 2 , J. Mark Sutton 2 , Khondaker Miraz Rahman 1
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1021/acsinfecdis.7b00130 Paolo Andriollo 1 , Charlotte K. Hind 2 , Pietro Picconi 1 , Kazi S. Nahar 1 , Shirin Jamshidi 1 , Amrit Varsha 1 , Melanie Clifford 2 , J. Mark Sutton 2 , Khondaker Miraz Rahman 1
Affiliation
|
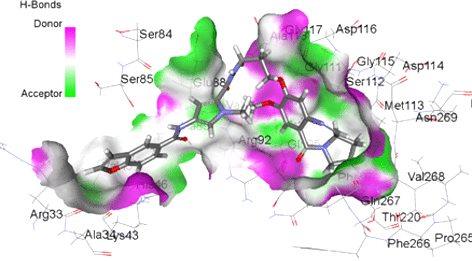
Antimicrobial resistance has become a major global concern. Development of novel antimicrobial agents for the treatment of infections caused by multidrug resistant (MDR) pathogens is an urgent priority. Pyrrolobenzodiazepines (PBDs) are a promising class of antibacterial agents initially discovered and isolated from natural sources. Recently, C8-linked PBD biaryl conjugates have been shown to be active against some MDR Gram-positive strains. To explore the role of building block orientations on antibacterial activity and obtain structure activity relationship (SAR) information, four novel structures were synthesized in which the building blocks of previously reported compounds were inverted, and their antibacterial activity was studied. The compounds showed minimum inhibitory concentrations (MICs) in the range of 0.125–32 μg/mL against MDR Gram-positive strains with a bactericidal mode of action. The results showed that a single inversion of amide bonds reduces the activity while the double inversion restores the activity against MDR pathogens. All inverted compounds did not stabilize DNA and lacked eukaryotic toxicity. The compounds inhibit DNA gyrase in vitro, and the most potent compound was equally active against both wild-type and mutant DNA gyrase in a biochemical assay. The observed activity of the compounds against methicillin resistant S. aureus (MRSA) strains with equivalent gyrase mutations is consistent with gyrase inhibition being the mechanism of action in vivo, although this has not been definitively confirmed in whole cells. This conclusion is supported by a molecular modeling study showing interaction of the compounds with wild-type and mutant gyrases. This study provides important SAR information about this new class of antibacterial agents.
中文翻译:

具有反向结构单元的C8连接的吡咯并苯并二氮杂Mono单体对多药耐药革兰氏阳性细菌表现出选择性活性
抗菌素耐药性已成为全球关注的主要问题。开发用于治疗由多药耐药性(MDR)病原体引起的感染的新型抗菌剂是当务之急。吡咯并苯二氮卓类(PBD)是一类有前途的抗菌剂,最初是从自然资源中发现和分离的。最近,C8连接的PBD联芳基共轭物已被证明对某些MDR革兰氏阳性菌株具有活性。为了探索结构单元取向对抗菌活性的作用并获得结构活性关系(SAR)信息,合成了四个新颖的结构,其中先前报道的化合物的结构单元被颠倒了,并研究了它们的抗菌活性。化合物的最低抑菌浓度(MIC)在0范围内。125-32μg/ mL,具有杀菌作用的MDR革兰氏阳性菌株。结果表明,酰胺键的一次反转降低了活性,而两次反转恢复了针对MDR病原体的活性。所有倒置的化合物都不能稳定DNA,并且缺乏真核毒性。这些化合物抑制DNA旋转酶在体外,在生化分析中,最有效的化合物对野生型和突变型DNA促旋酶均具有同等活性。所观察到的该化合物对具有等价回旋酶突变的耐甲氧西林金黄色葡萄球菌(MRSA)菌株的活性与回旋酶抑制作用在体内是一致的,尽管尚未在全细胞中明确证实。该结论得到分子模型研究的支持,该研究显示了化合物与野生型和突变型陀螺的相互作用。这项研究提供了有关这种新型抗菌剂的重要SAR信息。
更新日期:2018-01-09
中文翻译:

具有反向结构单元的C8连接的吡咯并苯并二氮杂Mono单体对多药耐药革兰氏阳性细菌表现出选择性活性
抗菌素耐药性已成为全球关注的主要问题。开发用于治疗由多药耐药性(MDR)病原体引起的感染的新型抗菌剂是当务之急。吡咯并苯二氮卓类(PBD)是一类有前途的抗菌剂,最初是从自然资源中发现和分离的。最近,C8连接的PBD联芳基共轭物已被证明对某些MDR革兰氏阳性菌株具有活性。为了探索结构单元取向对抗菌活性的作用并获得结构活性关系(SAR)信息,合成了四个新颖的结构,其中先前报道的化合物的结构单元被颠倒了,并研究了它们的抗菌活性。化合物的最低抑菌浓度(MIC)在0范围内。125-32μg/ mL,具有杀菌作用的MDR革兰氏阳性菌株。结果表明,酰胺键的一次反转降低了活性,而两次反转恢复了针对MDR病原体的活性。所有倒置的化合物都不能稳定DNA,并且缺乏真核毒性。这些化合物抑制DNA旋转酶在体外,在生化分析中,最有效的化合物对野生型和突变型DNA促旋酶均具有同等活性。所观察到的该化合物对具有等价回旋酶突变的耐甲氧西林金黄色葡萄球菌(MRSA)菌株的活性与回旋酶抑制作用在体内是一致的,尽管尚未在全细胞中明确证实。该结论得到分子模型研究的支持,该研究显示了化合物与野生型和突变型陀螺的相互作用。这项研究提供了有关这种新型抗菌剂的重要SAR信息。



























 京公网安备 11010802027423号
京公网安备 11010802027423号