当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Binding Specificity Determines the Cytochrome P450 3A4 Mediated Enantioselective Metabolism of Metconazole
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.jpcb.7b11170 Shulin Zhuang 1 , Leili Zhang 2 , Tingjie Zhan 1 , Liping Lu 1, 3 , Lu Zhao 1 , Haifei Wang 1 , Joseph A. Morrone 2 , Weiping Liu 1 , Ruhong Zhou 2, 4
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2018-01-12 00:00:00 , DOI: 10.1021/acs.jpcb.7b11170 Shulin Zhuang 1 , Leili Zhang 2 , Tingjie Zhan 1 , Liping Lu 1, 3 , Lu Zhao 1 , Haifei Wang 1 , Joseph A. Morrone 2 , Weiping Liu 1 , Ruhong Zhou 2, 4
Affiliation
|
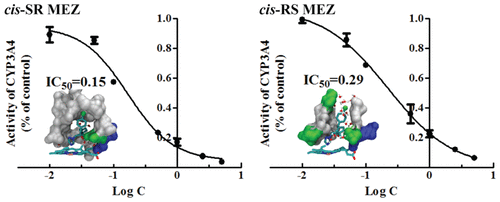
Cytochrome P450 3A4 (CYP3A4) is a promiscuous enzyme, mediating the biotransformations of ∼50% of clinically used drugs, many of which are chiral molecules. Probing the interactions between CYP3A4 and chiral chemicals is thus essential for the elucidation of molecular mechanisms of enantioselective metabolism. We developed a stepwise-restrained-molecular-dynamics (MD) method to model human CYP3A4 in a complex with cis-metconazole (MEZ) isomers and performed conventional MD simulations with a total simulation time of 2.2 μs to probe the molecular interactions. Our current study, which employs a combined experimental and theoretical approach, reports for the first time on the distinct conformational changes of CYP3A4 that are induced by the enantioselective binding of cis-MEZ enantiomers. CYP3A4 preferably metabolizes cis-RS MEZ over the cis-SR isomer, with the resultant enantiomer fraction for cis-MEZ increasing rapidly from 0.5 to 0.82. cis-RS MEZ adopts a more extended structure in the active pocket with its Cl atom exposed to the solvent, whereas cis-SR MEZ sits within the hydrophobic core of the active pocket. Free-energy-perturbation calculations indicate that unfavorable van der Waals interactions between the cis-MEZ isomers and the CYP3A4 binding pocket predominantly contribute to their binding-affinity differences. These results demonstrate that binding specificity determines the cytochrome P450 3A4 mediated enantioselective metabolism of cis-MEZ.
中文翻译:

结合特异性决定了Metconazole的细胞色素P450 3A4介导的对映选择性代谢
细胞色素P450 3A4(CYP3A4)是一种混杂酶,介导约50%的临床使用药物的生物转化,其中许多是手性分子。因此,探索CYP3A4与手性化学品之间的相互作用对于阐明对映选择性代谢的分子机制至关重要。我们开发了一种逐步约束分子动力学(MD)方法来模拟人CYP3A4与顺式-甲康唑(MEZ)异构体的复合物,并执行了传统的MD模拟,总模拟时间为2.2μs,以探测分子间的相互作用。我们目前的研究采用实验和理论相结合的方法,首次报道了顺式对映体选择性结合引起的CYP3A4构象变化。-MEZ对映异构体。CYP3A4代谢优选顺- RS MEZ在顺- SR异构体,与用于对映异构体得到的分数顺-MEZ迅速增加为0.5〜0.82。顺式- RS MEZ采用了一种更加延长结构与暴露于所述溶剂中的氯原子的活性口袋,而顺式- SR MEZ坐在活性袋的疏水性核内。自由能扰动计算表明顺式之间不利的范德华相互作用-MEZ异构体和CYP3A4结合口袋主要是造成它们的结合亲和力差异。这些结果证明结合特异性决定了细胞色素P450 3A4介导的顺式-MEZ对映选择性代谢。
更新日期:2018-01-12
中文翻译:

结合特异性决定了Metconazole的细胞色素P450 3A4介导的对映选择性代谢
细胞色素P450 3A4(CYP3A4)是一种混杂酶,介导约50%的临床使用药物的生物转化,其中许多是手性分子。因此,探索CYP3A4与手性化学品之间的相互作用对于阐明对映选择性代谢的分子机制至关重要。我们开发了一种逐步约束分子动力学(MD)方法来模拟人CYP3A4与顺式-甲康唑(MEZ)异构体的复合物,并执行了传统的MD模拟,总模拟时间为2.2μs,以探测分子间的相互作用。我们目前的研究采用实验和理论相结合的方法,首次报道了顺式对映体选择性结合引起的CYP3A4构象变化。-MEZ对映异构体。CYP3A4代谢优选顺- RS MEZ在顺- SR异构体,与用于对映异构体得到的分数顺-MEZ迅速增加为0.5〜0.82。顺式- RS MEZ采用了一种更加延长结构与暴露于所述溶剂中的氯原子的活性口袋,而顺式- SR MEZ坐在活性袋的疏水性核内。自由能扰动计算表明顺式之间不利的范德华相互作用-MEZ异构体和CYP3A4结合口袋主要是造成它们的结合亲和力差异。这些结果证明结合特异性决定了细胞色素P450 3A4介导的顺式-MEZ对映选择性代谢。


























 京公网安备 11010802027423号
京公网安备 11010802027423号