当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Recurrent supramolecular motifs in discrete complexes and coordination polymers based on mercury halides: prevalence of chelate ring stacking and substituent effects†
CrystEngComm ( IF 3.1 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1039/c7ce02166f Ghodrat Mahmoudi 1, 2, 3, 4, 5 , Jan K. Zaręba 6, 7, 8, 9 , Antonio Bauzá 10, 11, 12, 13 , Maciej Kubicki 9, 14, 15, 16 , Agata Bartyzel 9, 17, 18, 19 , Anastasios D. Keramidas 1, 20, 21, 22 , Leonid Butusov 23, 24, 25 , Barbara Mirosław 9, 14, 19, 26, 27 , Antonio Frontera 10, 11, 12, 13
CrystEngComm ( IF 3.1 ) Pub Date : 2018-01-09 00:00:00 , DOI: 10.1039/c7ce02166f Ghodrat Mahmoudi 1, 2, 3, 4, 5 , Jan K. Zaręba 6, 7, 8, 9 , Antonio Bauzá 10, 11, 12, 13 , Maciej Kubicki 9, 14, 15, 16 , Agata Bartyzel 9, 17, 18, 19 , Anastasios D. Keramidas 1, 20, 21, 22 , Leonid Butusov 23, 24, 25 , Barbara Mirosław 9, 14, 19, 26, 27 , Antonio Frontera 10, 11, 12, 13
Affiliation
|
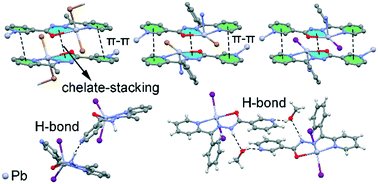
In recent years, the crystal engineering library has been enriched with a number of previously unrecognized or unnoticed intermolecular interactions, such as agostic, tetrel, chalcogen, pnicogen bonding and chelate ring stacking – collectively referred to as “unconventional interactions”. Many open questions remain unaddressed regarding their ability to form synthon interactions, specificity, and cooperativity, for example with π–π stacking interactions. In this work, we throw light on the formation of chelate ring stacking in metal–organic assemblies of nicotinohydrazide ligands (N′-(1-(2-pyridyl)ethylidene)nicotinohydrazide (HL) and N′-(phenyl(pyridin-2-yl)methylene)nicotinohydrazide (HL1)) with mercury(II) halide (HgBr2, HgI2) salts. Their reaction produced five compounds, namely [Hg(μ-L)BrHgBr2]n (1), [Hg(μ-L1)Br]n (2), [Hg(L)I2] (3), [Hg(HL1)I2]·(CH3OH) (4), and [Hg(μ-L1)I]n (5). Crystal structure analysis reveals that chelate ring stackings are formed in four of the reported metal–organic compounds, and are common also in the literature precedents. The energies of chelate ring stackings and π–π heterocycle stackings have been computed and analyzed by means of DFT calculations, and the results were verified using Bader's theory of “atoms in molecules”. These results provide a rationale for preferential formation of both unconventional and conventional stackings and allow us to conclude that chelate ring interaction may be considered as a synthon interaction for nicotinohydrazide metal complexes. Interpretations for packing differences imposed by the substituent effect (substitution of methyl group in HL for phenyl group in HL1) were provided based on the Hirshfeld surface analysis and 2D fingerprint plots of the crystal structures reported here.
中文翻译:

基于卤化汞的离散配合物和配位聚合物中的反复超分子基序:螯合环堆积的普遍性和取代基的作用†
近年来,晶体工程库中充斥了许多以前无法识别或未注意到的分子间相互作用,例如异形,t,硫属元素,成色剂键合和螯合环堆积-统称为“非常规相互作用”。关于它们形成合成子相互作用,特异性和协同性的能力,例如π-π堆积相互作用,许多悬而未决的问题尚未得到解决。在这项工作中,我们阐明了烟酰肼配体(N '-(1-(2-吡啶基)亚乙基)烟酰肼(HL)和N '-(苯基(吡啶-2)含汞(-II)亚甲基)烟酰肼(HL 1)))卤(HgBr 2,HGI 2)的盐。其反应产生的五种化合物,即[汞(μ-L)BrHgBr 2 ] Ñ(1),[汞(μ-L 1)BR] Ñ(2),[汞柱(L)予2 ](3),[汞柱(HL 1)我2 ]·(CH 3 OH)(4),和[汞(μ-L 1)I] ñ(5)。晶体结构分析表明,螯合环堆积是在四个已报道的金属有机化合物中形成的,并且在文献先例中也很常见。螯合环堆积和π-π杂环堆积的能量已经通过DFT计算进行了计算和分析,并使用Bader的“分子中的原子”理论对结果进行了验证。这些结果为优先形成非常规和常规堆积提供了理论依据,并使我们得出结论,螯合环相互作用可被视为烟酰肼金属配合物的合成子相互作用。用于包装的差异的解释在施加由所述取代基效应(甲基取代HL为苯基HL 1)是根据Hirshfeld表面分析和此处报告的晶体结构的2D指纹图提供的。
更新日期:2018-01-09
中文翻译:

基于卤化汞的离散配合物和配位聚合物中的反复超分子基序:螯合环堆积的普遍性和取代基的作用†
近年来,晶体工程库中充斥了许多以前无法识别或未注意到的分子间相互作用,例如异形,t,硫属元素,成色剂键合和螯合环堆积-统称为“非常规相互作用”。关于它们形成合成子相互作用,特异性和协同性的能力,例如π-π堆积相互作用,许多悬而未决的问题尚未得到解决。在这项工作中,我们阐明了烟酰肼配体(N '-(1-(2-吡啶基)亚乙基)烟酰肼(HL)和N '-(苯基(吡啶-2)含汞(-II)亚甲基)烟酰肼(HL 1)))卤(HgBr 2,HGI 2)的盐。其反应产生的五种化合物,即[汞(μ-L)BrHgBr 2 ] Ñ(1),[汞(μ-L 1)BR] Ñ(2),[汞柱(L)予2 ](3),[汞柱(HL 1)我2 ]·(CH 3 OH)(4),和[汞(μ-L 1)I] ñ(5)。晶体结构分析表明,螯合环堆积是在四个已报道的金属有机化合物中形成的,并且在文献先例中也很常见。螯合环堆积和π-π杂环堆积的能量已经通过DFT计算进行了计算和分析,并使用Bader的“分子中的原子”理论对结果进行了验证。这些结果为优先形成非常规和常规堆积提供了理论依据,并使我们得出结论,螯合环相互作用可被视为烟酰肼金属配合物的合成子相互作用。用于包装的差异的解释在施加由所述取代基效应(甲基取代HL为苯基HL 1)是根据Hirshfeld表面分析和此处报告的晶体结构的2D指纹图提供的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号