当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solubility Modeling and Mixing Properties for Benzoin in Different Monosolvents and Solvent Mixtures at the Temperature Range from 273.15 to 313.15 K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1021/acs.jced.7b00743 Yanqing Zhu 1 , Chao Cheng 2 , Gaoquan Chen 2 , Hongkun Zhao 2
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1021/acs.jced.7b00743 Yanqing Zhu 1 , Chao Cheng 2 , Gaoquan Chen 2 , Hongkun Zhao 2
Affiliation
|
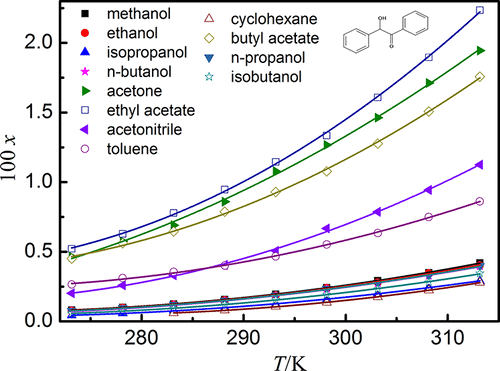
In the present work, the benzoin solubility in ethanol, methanol, n-propanol, isopropyl alcohol, n-butanol, acetone, ethyl acetate, acetonitrile, cyclohexane, butyl acetate, isobutyl alcohol, and toluene and ethyl acetate + ethanol solvent mixtures was measured by using the static method at the temperature range from 273.15 to 313.15 K under atmospheric pressure (101.1 kPa). The solubilities in mole fraction increased with increasing temperature and followed the order from high to low in the selected monosolvents: ethyl acetate > acetone > butyl acetate > (acetonitrile, toluene) > methanol > ethanol > n-propanol > n-butanol > isobutyl alcohol > isopropyl alcohol > cyclohexane; and for the ethyl acetate + ethanol mixture, the mole fraction solubilities of benzoin increased with the increase in temperature and ethyl acetate mass fraction. The obtained solubility of benzoin in neat solvents was correlated with the Apelblat equation, λh equation, and Wilson and NRTL models; and in solvent mixtures of ethyl acetate (w) + ethanol (1 – w), with the Jouyban–Acree, van’t Hoff–Jouyban–Acree and Apelblat–Jouyban–Acree models. The largest value of root-mean-square deviation was 4.02 × 10–4, and relative average deviation was 2.36 × 10–2. Furthermore, the mixing enthalpy, mixing Gibbs energy, mixing entropy, activity coefficients under infinitesimal concentration (γ1∞), and reduced excess enthalpy (H1E,∞) were deduced.
中文翻译:

在273.15至313.15 K的温度范围内,苯甲酸在不同的单溶剂和溶剂混合物中的溶解度建模和混合特性
在本工作中,测定了安息香在乙醇,甲醇,正丙醇,异丙醇,正丁醇,丙酮,乙酸乙酯,乙腈,环己烷,乙酸丁酯,异丁醇以及甲苯和乙酸乙酯+乙醇溶剂混合物中的溶解度通过在大气压力(101.1 kPa)下从273.15到313.15 K的温度范围内使用静态方法。摩尔分数的溶解度随温度的升高而增加,并且在所选的单溶剂中从高到低依次为:乙酸乙酯>丙酮>乙酸丁酯>(乙腈,甲苯)>甲醇>乙醇>正丙醇> n-丁醇>异丁醇>异丙醇>环己烷; 对于乙酸乙酯+乙醇混合物,安息香的摩尔分数溶解度随温度和乙酸乙酯质量分数的增加而增加。安息香在纯溶剂中的溶解度与Apelblat方程,λh方程以及Wilson和NRTL模型相关。以及在乙酸乙酯(w)+乙醇(1 – w)的溶剂混合物中,采用Jouyban–Acree模型,van't Hoff–Jouyban–Acree和Apelblat–Jouyban–Acree模型。均方根偏差的最大值为4.02×10 –4,相对平均偏差为2.36×10 –2。此外,混合焓,无穷小浓度下混合Gibbs能,混合熵,活度系数(γ 1 ∞),和减少过量的焓(ħ 1 E,∞)推导出。
更新日期:2018-01-08
中文翻译:

在273.15至313.15 K的温度范围内,苯甲酸在不同的单溶剂和溶剂混合物中的溶解度建模和混合特性
在本工作中,测定了安息香在乙醇,甲醇,正丙醇,异丙醇,正丁醇,丙酮,乙酸乙酯,乙腈,环己烷,乙酸丁酯,异丁醇以及甲苯和乙酸乙酯+乙醇溶剂混合物中的溶解度通过在大气压力(101.1 kPa)下从273.15到313.15 K的温度范围内使用静态方法。摩尔分数的溶解度随温度的升高而增加,并且在所选的单溶剂中从高到低依次为:乙酸乙酯>丙酮>乙酸丁酯>(乙腈,甲苯)>甲醇>乙醇>正丙醇> n-丁醇>异丁醇>异丙醇>环己烷; 对于乙酸乙酯+乙醇混合物,安息香的摩尔分数溶解度随温度和乙酸乙酯质量分数的增加而增加。安息香在纯溶剂中的溶解度与Apelblat方程,λh方程以及Wilson和NRTL模型相关。以及在乙酸乙酯(w)+乙醇(1 – w)的溶剂混合物中,采用Jouyban–Acree模型,van't Hoff–Jouyban–Acree和Apelblat–Jouyban–Acree模型。均方根偏差的最大值为4.02×10 –4,相对平均偏差为2.36×10 –2。此外,混合焓,无穷小浓度下混合Gibbs能,混合熵,活度系数(γ 1 ∞),和减少过量的焓(ħ 1 E,∞)推导出。


























 京公网安备 11010802027423号
京公网安备 11010802027423号