当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of 2,5-disubstituted oxazoles via cobalt(III)-catalyzed cross-coupling of N-pivaloyloxyamides and alkynes
Chemical Communications ( IF 4.9 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1039/c7cc08611c Xiaolong Yu 1, 2, 3, 4, 5 , Kehao Chen 1, 2, 3, 4, 5 , Qi Wang 1, 2, 3, 4, 5 , Wenjing Zhang 1, 2, 3, 4, 5 , Jin Zhu 1, 2, 3, 4, 5
Chemical Communications ( IF 4.9 ) Pub Date : 2018-01-08 00:00:00 , DOI: 10.1039/c7cc08611c Xiaolong Yu 1, 2, 3, 4, 5 , Kehao Chen 1, 2, 3, 4, 5 , Qi Wang 1, 2, 3, 4, 5 , Wenjing Zhang 1, 2, 3, 4, 5 , Jin Zhu 1, 2, 3, 4, 5
Affiliation
|
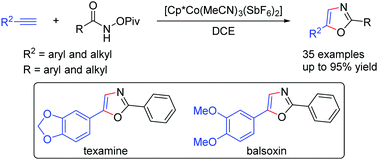
An efficient synthesis of 2,5-disubstituted oxazoles via Co(III) catalysis is described herein. The synthesis is achieved under mild conditions through [3+2] cycloaddition of N-pivaloyloxyamides and alkynes. The reaction operates through an internal oxidation pathway and features a very broad substrate scope. The one-step synthesis of natural products such as texamine and balsoxin has been demonstrated via this protocol.
中文翻译:

通过钴(III)催化的N-新戊酰氧基酰胺和炔烃的交叉偶联反应合成2,5-二取代的恶唑
本文描述了通过Co(III)催化的2,5-二取代的恶唑的有效合成。N-新戊酰氧基酰胺和炔烃的[3 + 2]环加成反应可在温和的条件下完成。该反应通过内部氧化途径进行,并且具有非常广泛的底物范围。通过该方案已证明了天然产品(如特克明和巴色辛)的一步合成。
更新日期:2018-01-31
中文翻译:

通过钴(III)催化的N-新戊酰氧基酰胺和炔烃的交叉偶联反应合成2,5-二取代的恶唑
本文描述了通过Co(III)催化的2,5-二取代的恶唑的有效合成。N-新戊酰氧基酰胺和炔烃的[3 + 2]环加成反应可在温和的条件下完成。该反应通过内部氧化途径进行,并且具有非常广泛的底物范围。通过该方案已证明了天然产品(如特克明和巴色辛)的一步合成。


























 京公网安备 11010802027423号
京公网安备 11010802027423号