当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Direct Stereoselective Installation of Alkyl Fragments at the β-Carbon of Enals via Excited Iminium Ion Catalysis
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acscatal.7b03788 Charlie Verrier 1 , Nurtalya Alandini 1 , Cristofer Pezzetta 1 , Mauro Moliterno 1 , Luca Buzzetti 1 , Hamish B. Hepburn 1 , Alberto Vega-Peñaloza 1 , Mattia Silvi 1 , Paolo Melchiorre 1, 2
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-01-10 00:00:00 , DOI: 10.1021/acscatal.7b03788 Charlie Verrier 1 , Nurtalya Alandini 1 , Cristofer Pezzetta 1 , Mauro Moliterno 1 , Luca Buzzetti 1 , Hamish B. Hepburn 1 , Alberto Vega-Peñaloza 1 , Mattia Silvi 1 , Paolo Melchiorre 1, 2
Affiliation
|
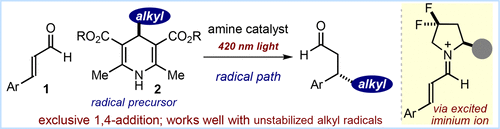
The direct introduction of sp3 carbon fragments at the β position of α,β-unsaturated aldehydes is greatly complicated by competing 1,2-addition manifolds. Previous catalytic enantioselective conjugate addition methods, based on the use of organometallic reagents or ground-state iminium ion activation, could not provide general and efficient solutions. We report herein that, by turning them into strong oxidants, visible light excitation of chiral iminium ions triggers a stereocontrolled radical pathway that exclusively affords highly enantioenriched β-substituted aldehydes bearing a variety of alkyl fragments.
中文翻译:

通过激动的离子离子催化在烯烃的β-碳上直接立体选择安装烷基片段
通过竞争1,2-加成歧管,在α,β-不饱和醛的β位置直接引入sp 3碳片段变得非常复杂。现有的基于使用有机金属试剂或基态亚胺离子活化的催化对映选择性共轭加成方法无法提供通用且有效的解决方案。我们在这里报道,通过将它们变成强氧化剂,手性亚胺离子的可见光激发触发了立体可控的自由基途径,该途径独家提供了带有各种烷基片段的高度对映体富集的β-取代的醛。
更新日期:2018-01-10
中文翻译:

通过激动的离子离子催化在烯烃的β-碳上直接立体选择安装烷基片段
通过竞争1,2-加成歧管,在α,β-不饱和醛的β位置直接引入sp 3碳片段变得非常复杂。现有的基于使用有机金属试剂或基态亚胺离子活化的催化对映选择性共轭加成方法无法提供通用且有效的解决方案。我们在这里报道,通过将它们变成强氧化剂,手性亚胺离子的可见光激发触发了立体可控的自由基途径,该途径独家提供了带有各种烷基片段的高度对映体富集的β-取代的醛。



























 京公网安备 11010802027423号
京公网安备 11010802027423号