当前位置:
X-MOL 学术
›
Anal. Methods
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Development and validation of an HPLC-FLD technique for colistin quantification and its plasma monitoring in hospitalized patients
Analytical Methods ( IF 3.1 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1039/c7ay02585h A. R. Pinho 1, 2, 3, 4, 5 , M. J. Rocha 5, 6 , G. Alves 5, 7, 8, 9 , A. C. Falcão 1, 2, 3, 4, 5 , A. C. Fortuna 1, 2, 3, 4, 5
Analytical Methods ( IF 3.1 ) Pub Date : 2018-01-05 00:00:00 , DOI: 10.1039/c7ay02585h A. R. Pinho 1, 2, 3, 4, 5 , M. J. Rocha 5, 6 , G. Alves 5, 7, 8, 9 , A. C. Falcão 1, 2, 3, 4, 5 , A. C. Fortuna 1, 2, 3, 4, 5
Affiliation
|
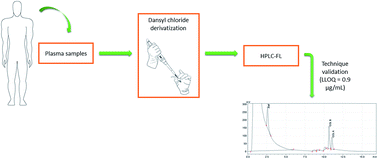
The increment of infections with multi-drug resistant gram-negative bacteria together with the high attrition rate of new antibacterial development programs has led to the renaissance of colistin as a new hope. However, the current administration of colistin to humans requires pharmacokinetic drug monitoring to individualize its posology, avoiding the development of resistant bacteria and the attainment of toxic concentrations. In this context, accurate, precise and selective methodologies are required to determine colistin plasma concentration. The present work is aimed at developing and fully validating a new high-performance liquid chromatography with fluorescence detection assay for the quantification of colistin in plasma samples of hospitalized patients. The chromatographic separation of colistin and an internal standard was achieved using a C18 column with a mobile phase comprised of acetonitrile and water. The detector was set at excitation/emission wavelengths of 343/500 nm and the retention time of the drug was shorter than those reported using other analytical techniques. The method was revealed to be linear in the concentration range of 0.09–9.00 μg mL−1 (which includes the therapeutic range of colistin), precise (coefficient of variance ≤ 6.4%), accurate (bias ≤ 14%) and selective. After full validation, the method successfully quantified the total colistin in plasma from patients treated with colistimethate sodium.
中文翻译:

用于住院患者大肠菌素定量和血浆监测的HPLC-FLD技术的开发和验证
具有多重耐药性的革兰氏阴性细菌感染的增加以及新的抗菌药物开发计划的高流失率,已使粘菌素的复兴成为新的希望。但是,目前向人施用大肠粘菌素需要对药代动力学药物进行监测,以使其个体化,避免耐药菌的产生和毒性浓度的达到。在这种情况下,需要精确,精确和选择性的方法来确定大肠菌素的血浆浓度。本工作旨在开发和完全验证一种新型的高效液相色谱仪,该仪器具有荧光检测测定法,用于定量住院患者血浆样品中的粘菌素。18柱,流动相由乙腈和水组成。该检测器的激发/发射波长设置为343/500 nm,药物的保留时间比使用其他分析技术报告的保留时间短。结果表明,该方法在0.09–9.00μgmL -1(包括大肠菌素的治疗范围)的浓度范围内是线性的,精确的(变异系数≤6.4%),准确的(偏差≤14%)和选择性的。经过充分验证后,该方法成功地定量了用大黄素钠处理过的患者血浆中的大肠粘菌素总量。
更新日期:2018-01-05
中文翻译:

用于住院患者大肠菌素定量和血浆监测的HPLC-FLD技术的开发和验证
具有多重耐药性的革兰氏阴性细菌感染的增加以及新的抗菌药物开发计划的高流失率,已使粘菌素的复兴成为新的希望。但是,目前向人施用大肠粘菌素需要对药代动力学药物进行监测,以使其个体化,避免耐药菌的产生和毒性浓度的达到。在这种情况下,需要精确,精确和选择性的方法来确定大肠菌素的血浆浓度。本工作旨在开发和完全验证一种新型的高效液相色谱仪,该仪器具有荧光检测测定法,用于定量住院患者血浆样品中的粘菌素。18柱,流动相由乙腈和水组成。该检测器的激发/发射波长设置为343/500 nm,药物的保留时间比使用其他分析技术报告的保留时间短。结果表明,该方法在0.09–9.00μgmL -1(包括大肠菌素的治疗范围)的浓度范围内是线性的,精确的(变异系数≤6.4%),准确的(偏差≤14%)和选择性的。经过充分验证后,该方法成功地定量了用大黄素钠处理过的患者血浆中的大肠粘菌素总量。



























 京公网安备 11010802027423号
京公网安备 11010802027423号