当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solid Parahydrogen Infrared Matrix Isolation and Computational Studies of Lin–(C2H4)m Complexes
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-01-19 00:00:00 , DOI: 10.1021/acs.jpca.7b11223 Laura F. Pinelo 1 , Elsbeth R. Klotz 1 , William R. Wonderly 1 , Leif O. Paulson 1 , Sharon C. Kettwich 1 , Jan Kubelka 1 , David T. Anderson 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-01-19 00:00:00 , DOI: 10.1021/acs.jpca.7b11223 Laura F. Pinelo 1 , Elsbeth R. Klotz 1 , William R. Wonderly 1 , Leif O. Paulson 1 , Sharon C. Kettwich 1 , Jan Kubelka 1 , David T. Anderson 1
Affiliation
|
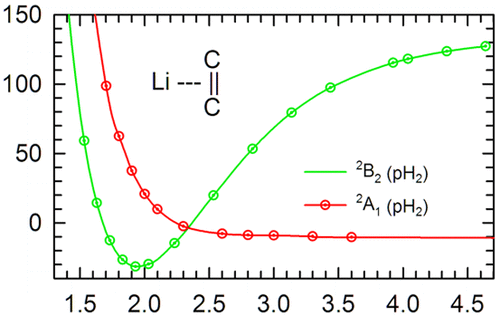
Complexes of lithium atoms with ethylene have been identified as potential hydrogen storage materials. As a Li atom approaches an ethylene molecule, two distinct low-lying electronic states are established; one is the 2A1 electronic state (for C2v geometries) that is repulsive but supports a shallow van der Waals well and correlates with the Li 2s atomic state, and the second is a 2B2 electronic state that correlates with the Li 2p atomic orbital and is a strongly bound charge-transfer state. Only the 2B2 charge-transfer state would be advantageous for hydrogen storage because the strong electric dipole created in the Li–(C2H4) complex due to charge transfer can bind molecular hydrogen through dipole–induced dipole and dipole–quadrupole electrostatic interactions. Ab initio studies have produced conflicting results for which electronic state is the true ground state for the Li–(C2H4) complex. The most accurate ab initio calculations indicate that the 2A1 van der Waals state is slightly more stable. In contrast, argon matrix isolation experiments have clearly identified the Li–(C2H4) complex exists in the 2B2 state. Some have suggested that argon matrix effects shift the equilibrium toward the 2B2 state. We report the low-temperature synthesis and IR characterization of Lin–(C2H4)m (n = 1, m = 1 and 2) complexes in solid parahydrogen which are observed using the C═C stretching vibration of ethylene in the complex. These results show that under cryogenic hydrogen storage conditions the Li–(C2H4) complex is more stable in the 2B2 electronic state and thus constitutes a potential hydrogen storage material with desirable characteristics.
中文翻译:

Li n –(C 2 H 4)m配合物的固体对氢红外矩阵分离和计算研究
锂原子与乙烯的配合物已被确定为潜在的储氢材料。当一个Li原子接近一个乙烯分子时,就会建立两个不同的低位电子态。一种是排斥的2 A 1电子态(对于C 2 v几何结构),但能很好地支撑浅范德华力,并与Li 2s原子态相关;第二种是与Li关联的2 B 2电子态。 2p原子轨道,是一种强束缚的电荷转移状态。只有2乙2电荷转移状态会储氢是有利的,因为在对锂产生的强电偶极子(C由于电荷转移引起的2 H 4)络合物可以通过偶极感应的偶极和偶极四极子的静电相互作用结合分子氢。从头算研究得出了矛盾的结果,其中电子状态是Li–(C 2 H 4)络合物的真正基态。最准确的从头算计算表明2 A 1 van der Waals状态稍微更稳定。相比之下,氩气基质分离实验已经清楚地确定了Li–(C 2 H 4)络合物存在于2 B 2中。状态。一些人认为,氩气基质效应使平衡向2 B 2状态移动。我们报道了在固态对氢中Li n –(C 2 H 4)m(n = 1,m = 1和2)配合物的低温合成和IR表征,这是通过乙烯在乙烯中的C═C拉伸振动观察到的。复杂的。这些结果表明,在低温储氢条件下,Li–(C 2 H 4)络合物在2 B 2电子状态下更稳定,因此构成了具有所需特性的潜在储氢材料。
更新日期:2018-01-19
中文翻译:

Li n –(C 2 H 4)m配合物的固体对氢红外矩阵分离和计算研究
锂原子与乙烯的配合物已被确定为潜在的储氢材料。当一个Li原子接近一个乙烯分子时,就会建立两个不同的低位电子态。一种是排斥的2 A 1电子态(对于C 2 v几何结构),但能很好地支撑浅范德华力,并与Li 2s原子态相关;第二种是与Li关联的2 B 2电子态。 2p原子轨道,是一种强束缚的电荷转移状态。只有2乙2电荷转移状态会储氢是有利的,因为在对锂产生的强电偶极子(C由于电荷转移引起的2 H 4)络合物可以通过偶极感应的偶极和偶极四极子的静电相互作用结合分子氢。从头算研究得出了矛盾的结果,其中电子状态是Li–(C 2 H 4)络合物的真正基态。最准确的从头算计算表明2 A 1 van der Waals状态稍微更稳定。相比之下,氩气基质分离实验已经清楚地确定了Li–(C 2 H 4)络合物存在于2 B 2中。状态。一些人认为,氩气基质效应使平衡向2 B 2状态移动。我们报道了在固态对氢中Li n –(C 2 H 4)m(n = 1,m = 1和2)配合物的低温合成和IR表征,这是通过乙烯在乙烯中的C═C拉伸振动观察到的。复杂的。这些结果表明,在低温储氢条件下,Li–(C 2 H 4)络合物在2 B 2电子状态下更稳定,因此构成了具有所需特性的潜在储氢材料。

























 京公网安备 11010802027423号
京公网安备 11010802027423号