当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adventures in Scaffold Morphing: Discovery of Fused Ring Heterocyclic Checkpoint Kinase 1 (CHK1) Inhibitors
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2018-01-19 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01490 Bin Yang 1 , Melissa M. Vasbinder 1 , Alexander W. Hird 1 , Qibin Su 1 , Haixia Wang 1 , Yan Yu 1 , Dorin Toader 1 , Paul D. Lyne 1 , Jon A. Read 2 , Jason Breed 2 , Stephanos Ioannidis 1 , Chun Deng 1 , Michael Grondine 1 , Nancy DeGrace 1 , David Whitston 1 , Patrick Brassil 1 , James W. Janetka 1
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2018-01-19 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01490 Bin Yang 1 , Melissa M. Vasbinder 1 , Alexander W. Hird 1 , Qibin Su 1 , Haixia Wang 1 , Yan Yu 1 , Dorin Toader 1 , Paul D. Lyne 1 , Jon A. Read 2 , Jason Breed 2 , Stephanos Ioannidis 1 , Chun Deng 1 , Michael Grondine 1 , Nancy DeGrace 1 , David Whitston 1 , Patrick Brassil 1 , James W. Janetka 1
Affiliation
|
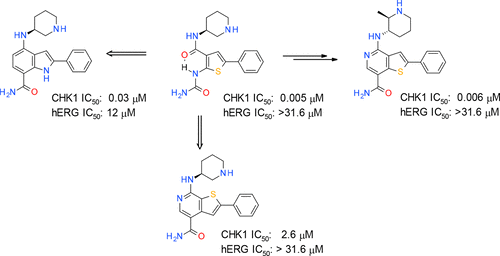
Checkpoint kinase 1 (CHK1) inhibitors are potential cancer therapeutics that can be utilized for enhancing the efficacy of DNA damaging agents. Multiple small molecule CHK1 inhibitors from different chemical scaffolds have been developed and evaluated in clinical trials in combination with chemotherapeutics and radiation treatment. Scaffold morphing of thiophene carboxamide ureas (TCUs), such as AZD7762 (1) and a related series of triazoloquinolines (TZQs), led to the identification of fused-ring bicyclic CHK1 inhibitors, 7-carboxamide thienopyridines (7-CTPs), and 7-carboxamide indoles. X-ray crystal structures reveal a key intramolecular noncovalent sulfur–oxygen interaction in aligning the hinge-binding carboxamide group to the thienopyridine core in a coplanar fashion. An intramolecular hydrogen bond to an indole NH was also effective in locking the carboxamide in the preferred bound conformation to CHK1. Optimization on the 7-CTP series resulted in the identification of lead compound 44, which displayed respectable drug-like properties and good in vitro and in vivo potency.
中文翻译:

脚手架变形历险记:融合环杂环检查点激酶1(CHK1)抑制剂的发现
Checkpoint激酶1(CHK1)抑制剂是潜在的癌症治疗剂,可用于增强DNA损伤剂的功效。已经开发了多种来自不同化学支架的小分子CHK1抑制剂,并在临床试验中结合化学疗法和放射治疗进行了评估。噻吩羧酰胺脲(TCU)的支架变形,例如AZD7762(1)和相关系列的三唑并喹啉(TZQs),导致鉴定了稠环双环CHK1抑制剂,7-羧酰胺噻吩并吡啶(7-CTP)和7-羧酰胺吲哚。X射线晶体结构揭示了以共面方式将铰链结合羧酰胺基团与噻吩并吡啶核对齐的关键分子内非共价硫-氧相互作用。与吲哚NH的分子内氢键也可有效地将羧酰胺锁定在与CHK1的优选结合构象中。对7-CTP系列进行优化后,鉴定出了铅化合物44,该化合物表现出可观的类药物特性以及良好的体外和体内药效。
更新日期:2018-01-19
中文翻译:

脚手架变形历险记:融合环杂环检查点激酶1(CHK1)抑制剂的发现
Checkpoint激酶1(CHK1)抑制剂是潜在的癌症治疗剂,可用于增强DNA损伤剂的功效。已经开发了多种来自不同化学支架的小分子CHK1抑制剂,并在临床试验中结合化学疗法和放射治疗进行了评估。噻吩羧酰胺脲(TCU)的支架变形,例如AZD7762(1)和相关系列的三唑并喹啉(TZQs),导致鉴定了稠环双环CHK1抑制剂,7-羧酰胺噻吩并吡啶(7-CTP)和7-羧酰胺吲哚。X射线晶体结构揭示了以共面方式将铰链结合羧酰胺基团与噻吩并吡啶核对齐的关键分子内非共价硫-氧相互作用。与吲哚NH的分子内氢键也可有效地将羧酰胺锁定在与CHK1的优选结合构象中。对7-CTP系列进行优化后,鉴定出了铅化合物44,该化合物表现出可观的类药物特性以及良好的体外和体内药效。


























 京公网安备 11010802027423号
京公网安备 11010802027423号