Water Research ( IF 12.8 ) Pub Date : 2018-01-05 , DOI: 10.1016/j.watres.2018.01.003 Jung Hwan Kim , Byung min An , Dae Hwan Lim , Joo Yang Park
|
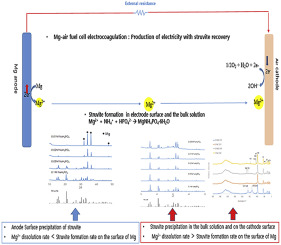
This research was based on the investigation of a major principle, regarding the effects of NaCl and KH2PO4 concentrations on struvite recovery, with electricity production using magnesium-air fuel cell electrocoagulation, in accordance with the concentration of phosphorous and chloride. The weight ratio of N:P in the synthetic wastewater was in the range of 1.2–21. The concentration of NH4Cl was fixed at 0.277 M (approximately 3888 ppm as NH3-N and 5000 ppm as NH4), while PO4-P was in the range of 0.006–0.1 M. In addition, the concentrations of NaCl as electrolyte were 0, 0.01, and 0.1 M. Phosphate removal increased linearly with the Mg:P ratio, up to approximately 1.1 mol mol−1, irrespective of the initial concentrations of phosphate and NaCl. The one-to-one reaction as mole ratio between phosphate and the dissolved Mg ions resulted in phosphate removal, with the production of a one-to-one magnesium/phosphate mineral, such as struvite. The dissolution rate of Mg ions with electro-oxidation determined the rate of phosphorous removal. The average removal rate of phosphorous in experiments without a dose of NaCl was 4.19 mg P cm−2 h−1, which was lower than the relative values of 5.35 and 4.77 mg P cm−2 h−1, in experiments with 0.01 and 0.1 M NaCl. The dissolution rate of Mg with electro-oxidation determined the rate of phosphorous removal with struvite recovery. The average removal rates of phosphorous with dose concentrations of 0.006, 0.01 and 0.02 M KH2PO4 were 4.02, 5.54, 6.9 mg P cm−2 h−1, respectively, which increased with the increase in KH2PO4 dose. However, in experiments with a dose of 0.05 and 0.1 M KH2PO4, the average removal rates of phosphorous decreased to 4.84 and 2.51, respectively. The maximum power densities in the electrolyte mixture of 0.05 M KH2PO4/0.277 M NH4Cl, 0.01 M NaCl/0.05 M KH2PO4/0.277 M NH4Cl, and 0.1 NaCl/0.05 KH2PO4/0.277 M NH4Cl were 25.1, 26.4, and 33.2 W/m2, respectively. The increase in the NaCl dose concentration resulted in an increase in the maximum power density and current density. A dose above 0.05 M KH2PO4 resulted in the decrease of the maximum power densities. However, when the dose was below 0.05 M KH2PO4, the maximum power density increased with the increase in KH2PO4 dose.
中文翻译:

镁空气燃料电池电凝法从合成废水中生成鸟粪石进行发电和磷回收
这项研究基于对主要原理的研究,涉及NaCl和KH 2 PO 4浓度对鸟粪石回收的影响,并根据镁和空气燃料电池的电凝作用,根据磷和氯的浓度进行发电。合成废水中N:P的重量比在1.2-21的范围内。NH的浓度4氯固定为0.277 M(约3888 ppm的作为NH 3 -N和5000ppm的作为NH 4),而PO 4 -P是0.006-0.1 M.另外的范围内,氯化钠的浓度电解质的去除量分别为0、0.01和0.1M。磷的去除率随Mg:P比例线性增加,最高达到约1.1 mol mol。-1,与磷酸盐和NaCl的初始浓度无关。磷酸根与溶解的Mg离子之间的摩尔比一对一反应可除去磷酸根,并生成一对一的镁/磷酸根矿物,例如鸟粪石。Mg离子在电氧化作用下的溶解速度决定了磷的去除速度。在不使用NaCl的实验中,磷的平均去除速率为4.19 mg P cm -2 h -1,低于5.35和4.77 mg P cm -2 h -1的相对值。在0.01和0.1 M NaCl的实验中。Mg在电氧化作用下的溶解速率决定了鸟粪石回收过程中磷的去除速率。剂量浓度为0.006、0.01和0.02 M KH 2 PO 4的磷的平均去除率分别为4.02、5.54、6.9 mg P cm -2 h -1,随KH 2 PO 4剂量的增加而增加。但是,在剂量为0.05和0.1 M KH 2 PO 4的实验中,磷的平均去除率分别降至4.84和2.51。电解液混合物中的最大功率密度为0.05 M KH 2 PO 4 /0.277 M NH4 Cl,0.01 M NaCl / 0.05 M KH 2 PO 4 /0.277 M NH 4 Cl和0.1 NaCl / 0.05 KH 2 PO 4 /0.277 M NH 4 Cl分别为25.1、26.4和33.2 W / m 2。NaCl剂量浓度的增加导致最大功率密度和电流密度的增加。高于0.05 M KH 2 PO 4的剂量导致最大功率密度的降低。但是,当剂量低于0.05 M KH 2 PO 4时,最大功率密度随KH 2 PO 4剂量的增加而增加。


























 京公网安备 11010802027423号
京公网安备 11010802027423号