Journal of Fluorine Chemistry ( IF 1.9 ) Pub Date : 2018-01-03 , DOI: 10.1016/j.jfluchem.2018.01.002 Ting Liu , Nan Yan , Hui Zhao , Zhen-Xing Wang , Xiang-Guo Hu
|
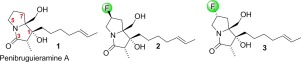
The synthesis of 6(R)- and 6(S)- fluoropenibruguieramine As has been achieved, employing the elegant strategy developed by Kim and co-workers. Single diastereomers were formed via the key intramolecular aldol reaction, and both of the products were unambiguously confirmed by X-ray diffraction crystallography. This reaction shows that the fluorine amide effect could not compete with the memory of chirality (MOC) effect, thus further demonstrating the powerfulness of MOC effect in asymmetric synthesis. The biological testing carried out in this work indicates that the principal of antibacterial activity of the natural extract is probably not penibruguieramine A.
中文翻译:

6(R)-和6(S)-氟代对苯二甲胺的合成及抗菌活性As:氟作为测试手性记忆(MOC)记忆力的探针
利用Kim和他的同事们开发的优美策略,已经完成了6(R)-和6(S)-氟代苯丙胺的合成。通过关键的分子内醛醇缩合反应形成单个非对映异构体,并且通过X射线衍射晶体学明确地证实了两种产物。该反应表明氟酰胺效应不能与手性(MOC)效应的记忆竞争,从而进一步证明了MOC效应在不对称合成中的强大作用。在这项工作中进行的生物学测试表明,天然提取物的抗菌活性的原理可能不是penibruguieramineA。



























 京公网安备 11010802027423号
京公网安备 11010802027423号