当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
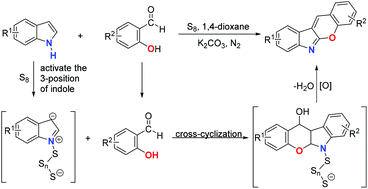
Elemental sulfur accelerated the reactivity of the 3-position of indole for the construction of chromeno[2,3-b]indoles†
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2018-01-04 00:00:00 , DOI: 10.1039/c7qo01114h Jianming Liu 1, 2, 3, 4, 5 , Xuyang Yan 1, 2, 3, 4, 5 , Na Liu 1, 2, 3, 4, 5 , Yanyan Zhang 1, 2, 3, 4, 5 , Shufang Zhao 1, 2, 3, 4, 5 , Xiaopei Wang 1, 2, 3, 4, 5 , Kelei Zhuo 1, 2, 3, 4, 5 , Yuanyuan Yue 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2018-01-04 00:00:00 , DOI: 10.1039/c7qo01114h Jianming Liu 1, 2, 3, 4, 5 , Xuyang Yan 1, 2, 3, 4, 5 , Na Liu 1, 2, 3, 4, 5 , Yanyan Zhang 1, 2, 3, 4, 5 , Shufang Zhao 1, 2, 3, 4, 5 , Xiaopei Wang 1, 2, 3, 4, 5 , Kelei Zhuo 1, 2, 3, 4, 5 , Yuanyuan Yue 1, 2, 3, 4, 5
Affiliation
|
An intermolecular cross-cyclization between salicylaldehydes and indoles for the preparation of chromeno[2,3-b]indole derivatives was successfully developed by using elemental sulfur as the promoter and oxidant. Various chromeno[2,3-b]indoles can be prepared using indoles and salicylaldehydes bearing different functional groups. The preliminary mechanistic investigations demonstrated that elemental sulfur not only acted as an additional oxidant but also facilitated the reactivity of the 3-position of indole.
中文翻译:

元素硫促进了吲哚3-位在铬诺[2,3- b ]吲哚结构上的反应性†
以元素硫为促进剂和氧化剂,成功开发了水杨醛与吲哚之间的分子间交叉环化反应,用于制备铬诺[2,3- b ]吲哚衍生物。可以使用带有不同官能团的吲哚和水杨醛制备各种chromeno [2,3- b ]吲哚。初步的机械研究表明,元素硫不仅充当了额外的氧化剂,而且还促进了吲哚3位的反应性。
更新日期:2018-01-04
中文翻译:

元素硫促进了吲哚3-位在铬诺[2,3- b ]吲哚结构上的反应性†
以元素硫为促进剂和氧化剂,成功开发了水杨醛与吲哚之间的分子间交叉环化反应,用于制备铬诺[2,3- b ]吲哚衍生物。可以使用带有不同官能团的吲哚和水杨醛制备各种chromeno [2,3- b ]吲哚。初步的机械研究表明,元素硫不仅充当了额外的氧化剂,而且还促进了吲哚3位的反应性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号