当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unlocking the potential of graphene for water oxidation using an orbital hybridization strategy†
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2018-01-04 00:00:00 , DOI: 10.1039/c7ee02972a Yingfang Yao 1, 2, 3, 4, 5 , Zhe Xu 1, 2, 3, 4, 5 , Feng Cheng 4, 5, 6, 7 , Wenchao Li 1, 2, 3, 4, 5 , Peixin Cui 7, 8, 9, 10, 11 , Guangzhou Xu 1, 2, 3, 4, 5 , Sheng Xu 2, 3, 4, 5, 7 , Peng Wang 2, 3, 4, 5, 7 , Guodong Sheng 6, 7, 12, 13 , Yadong Yan 1, 2, 3, 4, 5 , Zhentao Yu 1, 2, 3, 4, 5 , Shicheng Yan 1, 2, 3, 4, 5 , Zhaoxu Chen 4, 5, 6, 7 , Zhigang Zou 1, 2, 3, 4, 5
Energy & Environmental Science ( IF 32.5 ) Pub Date : 2018-01-04 00:00:00 , DOI: 10.1039/c7ee02972a Yingfang Yao 1, 2, 3, 4, 5 , Zhe Xu 1, 2, 3, 4, 5 , Feng Cheng 4, 5, 6, 7 , Wenchao Li 1, 2, 3, 4, 5 , Peixin Cui 7, 8, 9, 10, 11 , Guangzhou Xu 1, 2, 3, 4, 5 , Sheng Xu 2, 3, 4, 5, 7 , Peng Wang 2, 3, 4, 5, 7 , Guodong Sheng 6, 7, 12, 13 , Yadong Yan 1, 2, 3, 4, 5 , Zhentao Yu 1, 2, 3, 4, 5 , Shicheng Yan 1, 2, 3, 4, 5 , Zhaoxu Chen 4, 5, 6, 7 , Zhigang Zou 1, 2, 3, 4, 5
Affiliation
|
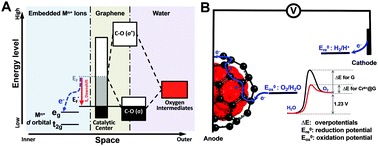
Graphene-based electrocatalytic materials are potential low-cost electrocatalysts for the oxygen evolution reaction (OER). However, substantial overpotentials above thermodynamic requirements limit their efficiency and stability in OER-related energy conversion and storage technologies. Here, we embedded CrN crystals into graphene and in situ electrochemically oxidized them to construct graphene materials with encapsulated Cr6+ ions (Cr6+@G). These Cr6+@G materials exhibit the lowest OER overpotential of 197 mV at 10 mA cm−2 and excellent stability over 200 h at a high current density of about 120 mA cm−2 in an alkaline electrolyte. Spectroscopic and computational studies confirm a stable ion coordination environment significantly benefiting the downshift of the graphene Fermi level via hybridization of C p orbitals with d orbitals of Cr6+ ions that enhances the OER activity and stability.
中文翻译:

使用轨道杂交策略释放石墨烯对水氧化的潜力†
石墨烯基电催化材料是用于氧释放反应(OER)的潜在低成本电催化剂。然而,超过热力学要求的大量超电势限制了它们在与OER相关的能量转换和存储技术中的效率和稳定性。在这里,我们将CrN晶体嵌入石墨烯中,并对其进行原位电化学氧化,以构建具有封装的Cr 6+离子(Cr 6+ @G)的石墨烯材料。这些Cr 6+ @G材料在10 mA cm -2时表现出最低的OER过电势,为197 mV,并且在约120 mA cm -2的高电流密度下,在200小时内具有出色的稳定性。在碱性电解液中。光谱和计算研究证实,稳定的离子配位环境通过Cp轨道与d 6 Cr离子的d轨道的杂交显着受益于石墨烯费米能级的下移,从而增强了OER活性和稳定性。
更新日期:2018-01-04
中文翻译:

使用轨道杂交策略释放石墨烯对水氧化的潜力†
石墨烯基电催化材料是用于氧释放反应(OER)的潜在低成本电催化剂。然而,超过热力学要求的大量超电势限制了它们在与OER相关的能量转换和存储技术中的效率和稳定性。在这里,我们将CrN晶体嵌入石墨烯中,并对其进行原位电化学氧化,以构建具有封装的Cr 6+离子(Cr 6+ @G)的石墨烯材料。这些Cr 6+ @G材料在10 mA cm -2时表现出最低的OER过电势,为197 mV,并且在约120 mA cm -2的高电流密度下,在200小时内具有出色的稳定性。在碱性电解液中。光谱和计算研究证实,稳定的离子配位环境通过Cp轨道与d 6 Cr离子的d轨道的杂交显着受益于石墨烯费米能级的下移,从而增强了OER活性和稳定性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号