European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2018-01-03 , DOI: 10.1016/j.ejmech.2017.12.098 A. Sergi Capilla , Richard Soucek , Laura Grau , Manel Romero , Jaime Rubio-Martínez , Daniel H. Caignard , Maria Dolors Pujol
|
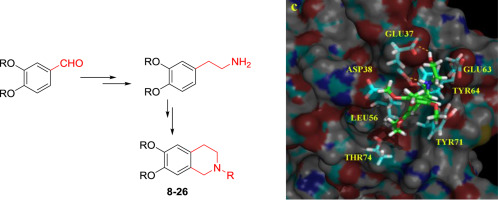
This work deals with the molecular design, synthesis and biological activity of a series of tetrahydro[1,4]dioxanisoquinolines and dimethoxyisoquinoline analogues. This study describes the synthesis strategy of these potential antitumor compounds, their multi-step synthesis and their optimization. A series of tetrahydroisoquinolines was synthesized and their cytotoxicity evaluated. Some of these tetrahydroisoquinolines showed promising KRas inhibition, antiangiogenesis activity and antiosteoporosis properties. Molecular modeling studies showed that compound 12 bind in the p1 pocket of the KRas protein making interactions with the hydrophobic residues Leu56, Tyr64, Tyr71 and Thr74 and hydrogen bonds with residues Glu37 and Asp38.
中文翻译:

取代的四氢异喹啉:合成,表征,抗肿瘤活性和其他生物学特性
这项工作涉及一系列四氢[1,4]二氧杂异喹啉和二甲氧基异喹啉类似物的分子设计,合成和生物活性。这项研究描述了这些潜在的抗肿瘤化合物的合成策略,它们的多步合成及其优化方法。合成了一系列四氢异喹啉并评估了它们的细胞毒性。这些四氢异喹啉中的一些表现出有希望的KRas抑制作用,抗血管生成活性和抗骨质疏松性质。分子模型研究表明,化合物12结合在KRas蛋白的p1口袋中,从而与疏水残基Leu56,Tyr64,Tyr71和Thr74相互作用,并与残基Glu37和Asp38形成氢键。



























 京公网安备 11010802027423号
京公网安备 11010802027423号