Applied Catalysis B: Environment and Energy ( IF 22.1 ) Pub Date : 2018-01-04 , DOI: 10.1016/j.apcatb.2018.01.001 Fuping Pan , Wei Deng , Carlos Justiniano , Ying Li
|
Transition metal is known to influence electrochemical activities over transition metals (M) and nitrogen (N)-codoped carbon (MN
C) catalysts. However, champion transition metals centers in M
N
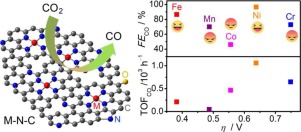
C for catalyzing CO2 reduction reaction (CO2RR) remain unclear, hindering further catalyst development with enhanced performance. Herein, we report the investigation of effects of five transition metals (Cr, Mn, Fe, Co, Ni) on CO2RR activities and mechanisms using metal-doped nitrogenated carbon nanosheets as model catalysts fabricated via a novel space-confinement-assisted molecular-level complexing approach. Analyzing N 1s XPS spectra confirmed the formation of M−N complexes via the coordination of metals atoms with pyridinic N, which was identified as the active species in CO2RR. According to activity descriptors including overpotentials, Faradaic efficiency (FE) and Turnover Frequency (TOF) per metal site, we here established that Fe and Ni are more active than Co, Mn, and Cr in M
N
C for the reduction of CO2 to CO. The main role of Fe is to reduce overpotentials, exhibiting the lowest onset overpotential of 0.19 V to yield CO on Fe
N
C. Ni can drastically improve CO selectivity and reaction rates, yielding the highest CO Faradaic efficiency of 96%, partial current density of −8.2 mA cm−2, and TOF of 1060 h−1 at a moderate overpotential of 0.65 V. Mechanism explorations reveal that CO2RR on M
N
C (M = Fe, Cr, Mn) undergoes the formation of a *COOH intermediate as the rate-determining step, whereas M
N
C (M = Ni, Co) catalyzes CO2RR via the transfer of the first electron to form a *CO2
− species. On the basis of the findings, we suggest doping Fe and/or Ni to design advanced M
N
C for CO2 electroreduction.
中文翻译:

在金属和氮共混碳催化剂中确定过渡金属中心的冠军,以还原CO 2
已知过渡金属会影响过渡金属(M)和氮(N)掺杂的碳(M N
C)催化剂的电化学活性。但是,冠军过渡金属位于M
N
C中心以催化CO 2还原反应(CO2RR)仍然不清楚,阻碍了进一步催化剂的开发,并提高了性能。在这里,我们报告的调查研究了五种过渡金属(Cr,Mn,Fe,Co,Ni)对CO2RR活性的影响以及使用掺杂金属的氮化碳纳米片作为模型催化剂的新型空间受限辅助分子级制备的机理。复杂化方法。分析N 1s XPS光谱证实了通过金属原子与吡啶N的配位形成了MN配合物,吡啶N被确定为CO2RR中的活性物种。根据包括每个金属位点的超电势,法拉第效率(FE)和周转频率(TOF)的活动描述符,我们在这里确定Fe和Ni在M
N
C中比Co,Mn和Cr更有活性,以减少CO 2的排放。Fe的主要作用是减少超电势,表现出最低的0.19 V起始超电势,以在Fe
N
C上生成CO。Ni可以极大地提高CO的选择性和反应速率,从而产生最高96%的CO法拉第效率。电流密度为-8.2 mA cm -2,TOF为1060 h -1,中度过电势为0.65V。机理探索表明,M
N
C(M = Fe,Cr,Mn)上的CO2RR经历了* COOH的形成中间体,为速率决定步骤,而中号
ñ
C(M =镍,钴)经由所述第一电子转移催化CO2RR以形成* CO 2
-物种。基于这些发现,我们建议对铁和/或镍进行掺杂以设计先进的M。
N
C用于CO 2电解还原。



























 京公网安备 11010802027423号
京公网安备 11010802027423号