Applied Catalysis B: Environment and Energy ( IF 22.1 ) Pub Date : 2018-01-04 , DOI: 10.1016/j.apcatb.2018.01.005 Yabo Wang , Jie Zhao , Yingxuan Li , Chuanyi Wang
|
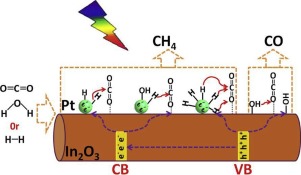
The selectivity of photocatalytic CO2 reduction to CH4 can be enhanced over Pt-decorated semiconductors, which is commonly attributed to the fact that a Schottky junction is formed, thus enhancing photoinduced electron lifetime. However, it is difficult to understand why the yield of CO—the other product of photocatalytic CO2 reduction—is decreased only in terms of photoinduced electron lifetime. In this work, the mechanism of Pt-promoted CH4 formation was probed again in a gas-solid system of photocatalytic CO2 reduction over highly-dispersed-Pt decorated In2O3 nanorods (Pt/In2O3). It was found that the presence of Pt modulates the surface property of In2O3 due to electronic and steric effect, resulting in a loss of HCO3−, b-CO32− and m-CO32− species for the coadsorption of CO2 with H2O. However, this is not directly related with the high CH4 selectivity and low CO yield on the Pt/In2O3 photocatalysts. Photocatalytic reductions of CO, HCOOH, CH2O, and CH3OH as well as photocatalytic CO2 reduction over photocatalysts with different H2 uptakes confirm that H adatoms derived from H2 or H2O dissociation on Pt play a key role in the formation of CH4. Low H2 dissociation barrier on Pt and weak HPt bond facilitate the bonding of C in CO2 with H, thus restraining CO production. In other words, the metallic Pt acts as atomic hydrogen reservoir that supplies sufficient and readily available protons for CH4 formation over Pt-decorated semiconductors. The present work offers a new window to explore non-noble metals or their alloys with stability in air and high dissociation ability to H2 or H2O as a replacement of Pt for CO2 photoreduction to CH4.
中文翻译:

Pt / In 2 O 3上选择性光催化将CO 2还原为CH 4:氢原子的重要作用
相较于用Pt装饰的半导体,可以提高光催化还原CO 2对CH 4的选择性,这通常归因于形成肖特基结的事实,从而提高了光致电子寿命。但是,很难理解为什么仅光诱导的电子寿命就降低了CO的收率(光催化还原CO 2的另一种产物)。在这项工作中,在高分散的Pt装饰的In 2 O 3纳米棒(Pt / In 2 O 3)上的光催化CO 2还原的气固系统中,再次探讨了Pt促进CH 4形成的机理。)。我们发现,Pt的存在下调节在表面属性2 ö 3由于电子和空间位阻效应,导致HCO损失3 -,B-CO 3 2-和M-CO 3 2-物种的共吸附CO 2与H 2 O混合。然而,这与Pt / In 2 O 3光催化剂上的高CH 4选择性和低CO收率没有直接关系。与具有不同H的光催化剂相比,CO,HCOOH,CH 2 O和CH 3 OH的光催化还原以及CO 2的光催化还原2次吸收证实,源自H 2或H 2 O在Pt上解离的H原子在CH 4的形成中起关键作用。Pt上的低H 2解离势垒和弱H Pt键促进了CO 2中的C与H的键合,从而抑制了CO的产生。换句话说,金属Pt充当原子氢储库,可为装饰Pt的半导体上的CH 4形成提供足够且容易获得的质子。本工作为探索在空气中具有稳定性且对H 2或H 2 O具有高离解能力以取代Pt替代CO 2的非贵金属或其合金提供了新的窗口。光还原为CH 4。



























 京公网安备 11010802027423号
京公网安备 11010802027423号