当前位置:
X-MOL 学术
›
ACS Biomater. Sci. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Anchoring of Iron Oxyhydroxide Clusters at H and L Ferritin Subunits
ACS Biomaterials Science & Engineering ( IF 5.8 ) Pub Date : 2018-01-23 00:00:00 , DOI: 10.1021/acsbiomaterials.7b00814 Steffen Lid 1 , Daniel Carmona 2 , Michael Maas 2 , Laura Treccani 2 , Lucio Colombi Ciacchi 1, 3
ACS Biomaterials Science & Engineering ( IF 5.8 ) Pub Date : 2018-01-23 00:00:00 , DOI: 10.1021/acsbiomaterials.7b00814 Steffen Lid 1 , Daniel Carmona 2 , Michael Maas 2 , Laura Treccani 2 , Lucio Colombi Ciacchi 1, 3
Affiliation
|
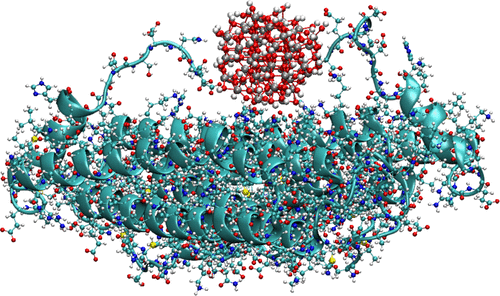
Ferritin (Fn) proteins or their isolated subunits can be used as biomolecular templates for the selectively heterogeneous nucleation and growth of nanoparticles, in particular of iron oxyhydroxides. To shed light on the atomistic mechanisms of ferritin-promoted mineralization, in this study we perform molecular dynamics simulations to investigate the anchoring sites for Fe(III) clusters on Fn subunit assemblies using models of goethite and ferrihydrite nanoparticles. For this aim, we develop and parametrize a classical force field for Fe(III) oxyhydroxides based on reference density functional theory calculations. We then reveal that stable Fn–nanoparticle contacts are formed not only via negatively charged amino acid residues (glutamic and aspartic acid) but also, in a similar amount, via positively charged (lysine and arginine) and neutral (histidine) residues. A large majority of the anchoring sites are situated at the inner side of protein cages, consistent with the natural iron storage function of ferritin in many organisms. A slightly different distribution of anchoring sites is observed on heavy (H) and light (L) Fn subunits, with the former offering a larger amount of negative and neutral sites than the latter. This finding is exploited to develop a Fn mineralization protocol in which immobilized Fn subunits are first loaded with Fe2+ ions in a long “activation” step before starting their oxidation to Fe3+. This leads to the formation of very dense and uniform iron oxide films, especially when H subunits are employed.
中文翻译:

铁和羟基铁蛋白亚基上的羟基氧化铁簇的锚定
铁蛋白(Fn)蛋白或其分离的亚基可以用作生物分子模板,用于纳米颗粒,特别是羟基氧化铁的选择性异质成核和生长。为了阐明铁蛋白促进矿化的原子机理,在这项研究中,我们进行了分子动力学模拟,以使用针铁矿和三水铁矿纳米颗粒模型研究Fn亚基组装体上Fe(III)团簇的锚固位点。为此,我们根据参考密度泛函理论计算结果开发并参数化了氢氧化三价铁(III)的经典力场。然后,我们揭示出稳定的Fn-纳米粒子接触不仅通过带负电荷的氨基酸残基(谷氨酸和天冬氨酸)形成,而且数量相似,通过带正电的(赖氨酸和精氨酸)和中性的(组氨酸)残基。大部分锚固位点位于蛋白笼的内侧,这与铁蛋白在许多生物中的天然铁存储功能一致。在重(H)和轻(L)Fn亚基上观察到锚点的分布略有不同,前者提供的负和中性位点比后者大。利用这一发现来开发Fn矿化方案,在该方案中,首先将固定化的Fn亚基加载Fe 前者提供的负面和中立网站数量要多于后者。利用这一发现来开发Fn矿化方案,在该方案中,首先将固定化的Fn亚基加载Fe 前者提供的负面和中立网站数量要多于后者。利用这一发现来开发Fn矿化方案,在该方案中,首先将固定化的Fn亚基加载Fe2+离子需要很长的“活化”步骤,才能开始氧化为Fe 3+。这导致形成非常致密且均匀的氧化铁膜,尤其是在使用H亚单元时。
更新日期:2018-01-23
中文翻译:

铁和羟基铁蛋白亚基上的羟基氧化铁簇的锚定
铁蛋白(Fn)蛋白或其分离的亚基可以用作生物分子模板,用于纳米颗粒,特别是羟基氧化铁的选择性异质成核和生长。为了阐明铁蛋白促进矿化的原子机理,在这项研究中,我们进行了分子动力学模拟,以使用针铁矿和三水铁矿纳米颗粒模型研究Fn亚基组装体上Fe(III)团簇的锚固位点。为此,我们根据参考密度泛函理论计算结果开发并参数化了氢氧化三价铁(III)的经典力场。然后,我们揭示出稳定的Fn-纳米粒子接触不仅通过带负电荷的氨基酸残基(谷氨酸和天冬氨酸)形成,而且数量相似,通过带正电的(赖氨酸和精氨酸)和中性的(组氨酸)残基。大部分锚固位点位于蛋白笼的内侧,这与铁蛋白在许多生物中的天然铁存储功能一致。在重(H)和轻(L)Fn亚基上观察到锚点的分布略有不同,前者提供的负和中性位点比后者大。利用这一发现来开发Fn矿化方案,在该方案中,首先将固定化的Fn亚基加载Fe 前者提供的负面和中立网站数量要多于后者。利用这一发现来开发Fn矿化方案,在该方案中,首先将固定化的Fn亚基加载Fe 前者提供的负面和中立网站数量要多于后者。利用这一发现来开发Fn矿化方案,在该方案中,首先将固定化的Fn亚基加载Fe2+离子需要很长的“活化”步骤,才能开始氧化为Fe 3+。这导致形成非常致密且均匀的氧化铁膜,尤其是在使用H亚单元时。


























 京公网安备 11010802027423号
京公网安备 11010802027423号