当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Gold(I)-Catalyzed Glycosylation with Glycosyl o-Alkynylbenzoates as Donors
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2018-01-03 00:00:00 , DOI: 10.1021/acs.accounts.7b00573 Biao Yu 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2018-01-03 00:00:00 , DOI: 10.1021/acs.accounts.7b00573 Biao Yu 1
Affiliation
|
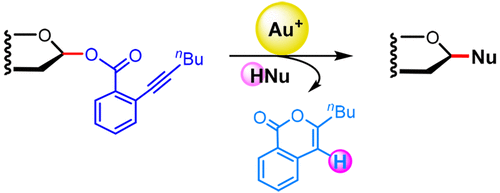
Naturally occurring glycans and glycoconjugates have extremely diverse structures and biological functions. Syntheses of these molecules and their artificial mimics, which have attracted the interest of those developing new therapeutic agents, rely on glycosylation methodologies to construct the various glycosidic linkages. In this regard, a wide array of glycosylation methods have been developed, and they mainly involve the substitution of a leaving group on the anomeric carbon of a glycosyl donor with an acceptor (a nucleophile) under the action of a particular promoter (usually a stoichiometric electrophile). However, glycosylations involving inherently unstable or unreactive donors/acceptors are still problematic. In those systems, reactions involving nucleophilic, electrophilic, or acidic species present on the leaving group and the promoter could become competitive and detrimental to the glycosylation. To address this problem, we applied the recently developed chemistry of alkynophilic gold(I) catalysts to the development of new glycosylation reactions that would avoid the use of the conventional leaving groups and promoters.
中文翻译:

金(I)催化的糖基邻烷基炔基苯甲酸酯作为供体的糖基化
天然存在的聚糖和糖缀合物具有极为不同的结构和生物学功能。这些分子及其人工模拟物的合成吸引了那些正在开发新治疗剂的人们的兴趣,它们依靠糖基化方法来构建各种糖苷键。在这方面,已经开发了各种各样的糖基化方法,并且它们主要涉及在特定启动子(通常是化学计量的)的作用下用受体(亲核试剂)取代糖基供体的异头碳上的离去基团。亲电)。然而,涉及固有不稳定或不反应的供体/受体的糖基化仍然是有问题的。在这些系统中,涉及亲核,亲电,存在于离去基团和启动子上的酸性物质可能对糖基化具有竞争性和有害性。为了解决这个问题,我们将最近开发的嗜碱金(I)催化剂化学方法用于开发新的糖基化反应,从而避免使用常规的离去基团和促进剂。
更新日期:2018-01-03
中文翻译:

金(I)催化的糖基邻烷基炔基苯甲酸酯作为供体的糖基化
天然存在的聚糖和糖缀合物具有极为不同的结构和生物学功能。这些分子及其人工模拟物的合成吸引了那些正在开发新治疗剂的人们的兴趣,它们依靠糖基化方法来构建各种糖苷键。在这方面,已经开发了各种各样的糖基化方法,并且它们主要涉及在特定启动子(通常是化学计量的)的作用下用受体(亲核试剂)取代糖基供体的异头碳上的离去基团。亲电)。然而,涉及固有不稳定或不反应的供体/受体的糖基化仍然是有问题的。在这些系统中,涉及亲核,亲电,存在于离去基团和启动子上的酸性物质可能对糖基化具有竞争性和有害性。为了解决这个问题,我们将最近开发的嗜碱金(I)催化剂化学方法用于开发新的糖基化反应,从而避免使用常规的离去基团和促进剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号