当前位置:
X-MOL 学术
›
J. Electroanal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Enhancing the lithium storage capacity of FeF 3 cathode material by introducing C@LiF additive
Journal of Electroanalytical Chemistry ( IF 4.5 ) Pub Date : 2018-02-01 , DOI: 10.1016/j.jelechem.2018.01.002 Xiangyang Zhou , Hongxu Sun , Haochen Zhou , Jing Ding , Zhanglin Xu , Wenjie Bin , Jingjing Tang , Juan Yang
Journal of Electroanalytical Chemistry ( IF 4.5 ) Pub Date : 2018-02-01 , DOI: 10.1016/j.jelechem.2018.01.002 Xiangyang Zhou , Hongxu Sun , Haochen Zhou , Jing Ding , Zhanglin Xu , Wenjie Bin , Jingjing Tang , Juan Yang
|
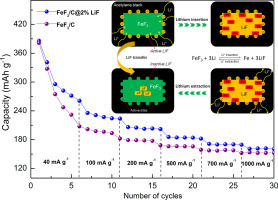
Abstract Iron fluorides based on conversion chemistry are developed as alternative cathode materials for Li-ion batteries due to their high capacity, also suffering from some drawbacks, such as poor electrical conductivity and structural instability. In order to improve the electrochemical performance of iron fluoride, in this work, we focus on the enhancement of the lithium extraction capability of FeF3 by introducing extra C@LiF additive. FeF3/C@LiF composites are fabricated by high energy ball milling. Due to the compact connection between LiF and conductive carbon in FeF3/C@LiF composites, LiF can not only act as high-active reactant for lithium extraction process, but also provide active sites for subsequent charge/discharge reaction. Electrochemical study results suggest that a suitable content of LiF enables FeF3/C@LiF composite to deliver a satisfactory electrochemical performance, which retain a capacity of 229 mAh g− 1 at a current rate of 40 mA g− 1 after 50 cycles (10% higher than that of FeF3/C composite).
中文翻译:

通过引入 C@LiF 添加剂提高 FeF 3 正极材料的锂存储容量
摘要 基于转化化学的氟化铁由于其高容量而被开发为锂离子电池的替代正极材料,但也存在一些缺点,如导电性差和结构不稳定。为了提高氟化铁的电化学性能,在这项工作中,我们通过引入额外的 C@LiF 添加剂来提高 FeF3 的锂提取能力。FeF3/C@LiF 复合材料是通过高能球磨制备的。由于 FeF3/C@LiF 复合材料中 LiF 与导电碳之间的紧密连接,LiF 不仅可以作为锂提取过程的高活性反应物,还可以为后续的充放电反应提供活性位点。
更新日期:2018-02-01
中文翻译:

通过引入 C@LiF 添加剂提高 FeF 3 正极材料的锂存储容量
摘要 基于转化化学的氟化铁由于其高容量而被开发为锂离子电池的替代正极材料,但也存在一些缺点,如导电性差和结构不稳定。为了提高氟化铁的电化学性能,在这项工作中,我们通过引入额外的 C@LiF 添加剂来提高 FeF3 的锂提取能力。FeF3/C@LiF 复合材料是通过高能球磨制备的。由于 FeF3/C@LiF 复合材料中 LiF 与导电碳之间的紧密连接,LiF 不仅可以作为锂提取过程的高活性反应物,还可以为后续的充放电反应提供活性位点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号