当前位置:
X-MOL 学术
›
J. Mater. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A Friedländer route to 5,7-diazapentacenes†
Journal of Materials Chemistry C ( IF 6.4 ) Pub Date : 2018-01-03 00:00:00 , DOI: 10.1039/c7tc05057g Andrey V. Lunchev 1, 2, 3 , Vincent C. Hendrata 1, 2, 3 , Aparna Jaggi 1, 2, 3 , Samuel A. Morris 1, 2, 3 , Rakesh Ganguly 2, 4, 5, 6, 7 , Xiaoxuan Chen 2, 8, 9, 10 , Handong Sun 2, 8, 9, 10 , Andrew C. Grimsdale 1, 2, 3
Journal of Materials Chemistry C ( IF 6.4 ) Pub Date : 2018-01-03 00:00:00 , DOI: 10.1039/c7tc05057g Andrey V. Lunchev 1, 2, 3 , Vincent C. Hendrata 1, 2, 3 , Aparna Jaggi 1, 2, 3 , Samuel A. Morris 1, 2, 3 , Rakesh Ganguly 2, 4, 5, 6, 7 , Xiaoxuan Chen 2, 8, 9, 10 , Handong Sun 2, 8, 9, 10 , Andrew C. Grimsdale 1, 2, 3
Affiliation
|
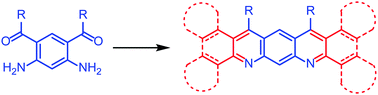
A route to compounds with a 5,7-diazapentacene skeleton has been established involving a Friedländer reaction. A diaminodiketone 8 has been made by a novel method and reacted with cyclohexanone to prepare an octa-hydro-5,7-diazapentacene 7a and with tetralone to produce a dibenzotetrahydro-5,7-diazapentacene 7b. Reaction of the diaminodiketone with a diarylethanone followed by oxidation gave a tetrabenzo-5,7-diazapentacene 18b. Compound 7b undergoes solid-state dimerization during single-crystal X-ray analysis. These materials possess lower frontier orbitals than pentacene and show strong absorption and fluorescence which is affected by the presence of acid. In particular 18b shows a remarkable colour change in solution upon addition of acid. These results suggest that suitably functionalised 5,7-diazapentacenes could be promising candidates for optoelectronic applications.
中文翻译:

弗里德兰德通往5,7-二氮杂戊烷的路线†
已经建立了涉及5,7-二氮杂戊并五烯骨架的化合物的方法,该方法涉及Friedländer反应。甲diaminodiketone 8已经取得了一种新颖的方法和与环己酮反应,制备八氢-5,7- diazapentacene 7A并与四氢萘酮以产生dibenzotetrahydro -5,7- diazapentacene 7B。二氨基二酮与二芳基酮的反应,然后氧化,得到四苯并-5,7-二氮杂戊并苯18b。化合物7b在单晶X射线分析过程中经历固态二聚化。这些材料比并五苯具有更低的前沿轨道,并且显示出强烈的吸收和荧光,这受酸的存在影响。特别是18b加入酸后溶液显示出显着的颜色变化。这些结果表明,适当官能化的5,7-二氮杂戊烷可以成为光电子应用的有前途的候选者。
更新日期:2018-01-03
中文翻译:

弗里德兰德通往5,7-二氮杂戊烷的路线†
已经建立了涉及5,7-二氮杂戊并五烯骨架的化合物的方法,该方法涉及Friedländer反应。甲diaminodiketone 8已经取得了一种新颖的方法和与环己酮反应,制备八氢-5,7- diazapentacene 7A并与四氢萘酮以产生dibenzotetrahydro -5,7- diazapentacene 7B。二氨基二酮与二芳基酮的反应,然后氧化,得到四苯并-5,7-二氮杂戊并苯18b。化合物7b在单晶X射线分析过程中经历固态二聚化。这些材料比并五苯具有更低的前沿轨道,并且显示出强烈的吸收和荧光,这受酸的存在影响。特别是18b加入酸后溶液显示出显着的颜色变化。这些结果表明,适当官能化的5,7-二氮杂戊烷可以成为光电子应用的有前途的候选者。


























 京公网安备 11010802027423号
京公网安备 11010802027423号