当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Copper-catalyzed peri-selective direct sulfenylation of 1-naphthylamines with disulfides†
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2018-01-03 00:00:00 , DOI: 10.1039/c7qo01016h Yan-Shi Xiong 1, 2, 3, 4, 5 , Yang Yu 1, 2, 3, 4, 5 , Jiang Weng 1, 2, 3, 4, 5 , Gui Lu 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2018-01-03 00:00:00 , DOI: 10.1039/c7qo01016h Yan-Shi Xiong 1, 2, 3, 4, 5 , Yang Yu 1, 2, 3, 4, 5 , Jiang Weng 1, 2, 3, 4, 5 , Gui Lu 1, 2, 3, 4, 5
Affiliation
|
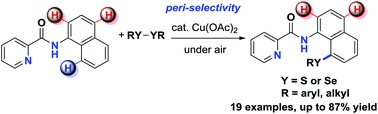
A copper-catalyzed peri-selective direct C–H sulfenylation of 1-naphthylamines with disulfides was developed. This protocol employs inexpensive Cu(OAc)2 as the catalyst, air as the terminal oxidant, and readily available diaryl (or dialkyl) disulfide and diselenide as chalcogenation reagents. High functional group tolerance and excellent regioselectivity were demonstrated by the efficient preparation of a wide range of 8-sulfenyl-1-naphthylamines.
中文翻译:

铜催化围-选择性的1 -萘胺的直接亚磺酰化与二硫化物†
铜-催化的迫-选择性直接C-H 1-萘胺与二硫化物亚磺酰化的开发。该方案使用廉价的Cu(OAc)2作为催化剂,使用空气作为末端氧化剂,并容易获得二芳基(或二烷基)二硫化物和二硒化物作为硫属化试剂。高官能团的耐受性和出色的区域选择性通过有效制备各种8-亚磺酰基-1-萘胺得到了证明。
更新日期:2018-01-03
中文翻译:

铜催化围-选择性的1 -萘胺的直接亚磺酰化与二硫化物†
铜-催化的迫-选择性直接C-H 1-萘胺与二硫化物亚磺酰化的开发。该方案使用廉价的Cu(OAc)2作为催化剂,使用空气作为末端氧化剂,并容易获得二芳基(或二烷基)二硫化物和二硒化物作为硫属化试剂。高官能团的耐受性和出色的区域选择性通过有效制备各种8-亚磺酰基-1-萘胺得到了证明。


























 京公网安备 11010802027423号
京公网安备 11010802027423号