当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Gas-Phase Oxidation of the Protonated Uracil-5-yl Radical Cation
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.jpca.7b09411 James P. Bezzina 1 , Matthew B. Prendergast 1 , Stephen J. Blanksby 2 , Adam J. Trevitt 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-01-17 00:00:00 , DOI: 10.1021/acs.jpca.7b09411 James P. Bezzina 1 , Matthew B. Prendergast 1 , Stephen J. Blanksby 2 , Adam J. Trevitt 1
Affiliation
|
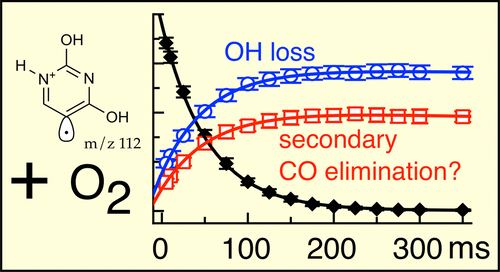
This study targets the kinetics and product detection of the gas-phase oxidation reaction of the protonated 5-dehydrouracil (uracil-5-yl) distonic radical cation using ion-trap mass spectrometry. Protonated 5-dehydrouracil radical ions (5-dehydrouracilH+ radical ion, m/z 112) are produced within an ion trap by laser photolysis of protonated 5-iodouracil. Storage of the 5-dehydrouracilH+ radical ion in the presence of controlled concentration of O2 reveals two main products. The major reaction product pathway is assigned as the formation of protonated 2-hydroxypyrimidine-4,5-dione (m/z 127) + •OH. A second product ion (m/z 99), putatively assigned as a five-member-ring ketone structure, is tentatively explained as arising from the decarbonylation (−CO) of protonated 2-hydroxypyrimidine-4,5-dione. Because protonation of the 5-dehydrouracil radical likely forms a dienol structure, the O2 reaction at the 5 position is ortho to an −OH group. Following this addition of O2, the peroxyl-radical intermediate isomerizes by H atom transfer from the −OH group. The ensuing hydroperoxide then decomposes to eliminate •OH radical. It is shown that this elimination of •OH radical (−17 Da) is evidence for the presence of an −OH group ortho to the initial phenyl radical site, in good accord with calculations. The subsequent CO loss mechanism, to form the aforementioned five-member-ring structure, is unclear, but some pathways are discussed. By following the kinetics of the reaction, the room temperature second-order rate coefficient of the 5-dehydrouracilH+ distonic radical cation with molecular oxygen is measured at 7.2 × 10–11 cm3 molecule–1 s–1, Φ = 12% (with ±50% total accuracy). For aryl radical reactions with O2, the presence of the •OH elimination product pathway, following the peroxyl-radical formation, is an indicator of an −OH group ortho to the radical site.
中文翻译:

质子化的尿嘧啶-5-基自由基阳离子的气相氧化
这项研究的目标是使用离子阱质谱法对质子化的5-脱氢尿嘧啶(尿嘧啶-5-基)二氢自由基阳离子的气相氧化反应进行动力学和产物检测。通过激光光解质子化的5-碘尿嘧啶,在离子阱内产生质子化的5-脱氢尿嘧啶自由基离子(5-脱氢尿嘧啶H +自由基离子,m / z 112)。在控制浓度的O 2存在下,5-dehydrouracilH +自由基离子的存储显示出两种主要产物。主要反应产物途径为形成质子化的2-羟基嘧啶-4,5-二酮(m / z 127)+ • OH。第二产物离子(m / z 99),被推定为五元环酮结构,初步解释为质子化的2-羟基嘧啶-4,5-二酮的脱羰作用(-CO)。因为5-脱氢尿嘧啶基的质子化可能形成二烯醇结构,所以在5位的O 2反应与-OH基邻位。在添加O 2之后,过氧自由基基中间体通过从-OH基团转移H原子而异构化。随后产生的氢过氧化物分解以消除• OH自由基。结果表明,• OH自由基(−17 Da)的消除是-OH邻位基团存在的证据。与最初的苯基自由基位点相符,与计算结果非常吻合。形成上述五元环结构的随后的CO损失机理尚不清楚,但是讨论了一些途径。通过反应动力学,测定了5-脱氢尿嘧啶H +异狄氏自由基阳离子与分子氧的室温二阶速率系数,测量值为7.2×10 –11 cm 3分子–1 s –1,Φ= 12%(总精度为±50%)。对于与O 2的芳基自由基反应,过氧自由基形成后• OH消除产物途径的存在是-OH基邻位的指示 到激进的网站。
更新日期:2018-01-17
中文翻译:

质子化的尿嘧啶-5-基自由基阳离子的气相氧化
这项研究的目标是使用离子阱质谱法对质子化的5-脱氢尿嘧啶(尿嘧啶-5-基)二氢自由基阳离子的气相氧化反应进行动力学和产物检测。通过激光光解质子化的5-碘尿嘧啶,在离子阱内产生质子化的5-脱氢尿嘧啶自由基离子(5-脱氢尿嘧啶H +自由基离子,m / z 112)。在控制浓度的O 2存在下,5-dehydrouracilH +自由基离子的存储显示出两种主要产物。主要反应产物途径为形成质子化的2-羟基嘧啶-4,5-二酮(m / z 127)+ • OH。第二产物离子(m / z 99),被推定为五元环酮结构,初步解释为质子化的2-羟基嘧啶-4,5-二酮的脱羰作用(-CO)。因为5-脱氢尿嘧啶基的质子化可能形成二烯醇结构,所以在5位的O 2反应与-OH基邻位。在添加O 2之后,过氧自由基基中间体通过从-OH基团转移H原子而异构化。随后产生的氢过氧化物分解以消除• OH自由基。结果表明,• OH自由基(−17 Da)的消除是-OH邻位基团存在的证据。与最初的苯基自由基位点相符,与计算结果非常吻合。形成上述五元环结构的随后的CO损失机理尚不清楚,但是讨论了一些途径。通过反应动力学,测定了5-脱氢尿嘧啶H +异狄氏自由基阳离子与分子氧的室温二阶速率系数,测量值为7.2×10 –11 cm 3分子–1 s –1,Φ= 12%(总精度为±50%)。对于与O 2的芳基自由基反应,过氧自由基形成后• OH消除产物途径的存在是-OH基邻位的指示 到激进的网站。



























 京公网安备 11010802027423号
京公网安备 11010802027423号