Chemical Physics ( IF 2.3 ) Pub Date : 2018-01-03 , DOI: 10.1016/j.chemphys.2018.01.001 Sara Hany , Mira Skaf , Samer Aouad , Cédric Gennequin , Madona Labaki , Edmond Abi-Aad , Antoine Aboukaïs
|
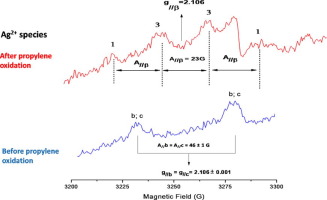
Three different types of Ag2+ ions (“a”, “b”, and “c”) have been identified and examined by electron paramagnetic resonance (EPR) on 10% wt Ag/CeO2 prepared by impregnation method. One of them, Ag2+(b), behaves differently than the two others, Ag2+(a) and Ag2+(c), under redox atmospheres. The fact that, in reducing conditions (vacuum, propylene, hydrogen, and carbon black), Ag2+(a) and Ag2+(c) species were more easily reduced than Ag2+(b) ones, could not explain the catalytic performance and stability of this latter species compared to the first ones in the reaction of total oxidation of propylene. The EPR technique evidenced that Ag2+(b) species form, upon propene oxidation, a cluster. This cluster is composed of two parallel electron spins (dimer) and three nuclear spins (trimer). It seems that before propylene oxidation, Ag2+(b) clusters were ferromagnetic. This ferromagnetic character of Ag2+(b) species may explain their better catalytic performance, in propylene oxidation, than those of Ag2+(a) and Ag2+(c) ones.
中文翻译:

负载在二氧化铈表面的Ag 2+团簇的尺寸和磁性与它们在丙烯的总氧化中的催化性能之间的相关性。EPR研究。
已经通过浸渍法制备的10%wt Ag / CeO 2的电子顺磁共振(EPR)鉴定并检查了三种不同类型的Ag 2+离子(“ a”,“ b”和“ c”)。其中一个Ag 2+ (b)在氧化还原气氛下的行为与另外两个Ag 2+ (a)和Ag 2+ (c)不同。在还原条件下(真空,丙烯,氢和炭黑),Ag 2+ (a)和Ag 2+ (c)物种比Ag 2+ (b)更容易还原的事实与第一个相比,它们不能解释在丙烯的全氧化反应中后一个物种的催化性能和稳定性。EPR技术证明,丙烯氧化后,Ag 2+ (b)物种形成簇。该簇由两个平行的电子自旋(二聚体)和三个核自旋(三聚体)组成。看来在丙烯氧化之前,Ag 2+ (b)团簇是铁磁性的。Ag 2+ (b)物种的这种铁磁特性可以解释其在丙烯氧化中比Ag 2+ (a)和Ag 2+ (c)更好的催化性能。


























 京公网安备 11010802027423号
京公网安备 11010802027423号