当前位置:
X-MOL 学术
›
Phytochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Anti-HIV and cytotoxic biphenyls, benzophenones and xanthones from stems, leaves and twigs of Garcinia speciosa
Phytochemistry ( IF 3.8 ) Pub Date : 2018-03-01 , DOI: 10.1016/j.phytochem.2017.12.013 Phanruethai Pailee , Chutima Kuhakarn , Chanyapat Sangsuwan , Sakchai Hongthong , Pawinee Piyachaturawat , Kanoknetr Suksen , Surawat Jariyawat , Radeekorn Akkarawongsapat , Jitra Limthongkul , Chanita Napaswad , Palangpon Kongsaeree , Samran Prabpai , Thaworn Jaipetch , Manat Pohmakotr , Patoomratana Tuchinda , Vichai Reutrakul
Phytochemistry ( IF 3.8 ) Pub Date : 2018-03-01 , DOI: 10.1016/j.phytochem.2017.12.013 Phanruethai Pailee , Chutima Kuhakarn , Chanyapat Sangsuwan , Sakchai Hongthong , Pawinee Piyachaturawat , Kanoknetr Suksen , Surawat Jariyawat , Radeekorn Akkarawongsapat , Jitra Limthongkul , Chanita Napaswad , Palangpon Kongsaeree , Samran Prabpai , Thaworn Jaipetch , Manat Pohmakotr , Patoomratana Tuchinda , Vichai Reutrakul
|
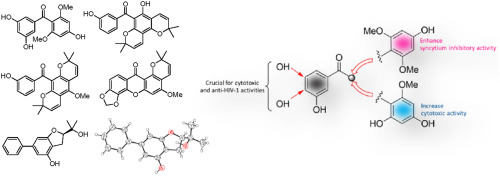
Eleven previously undescribed compounds, including four benzophenones (garciosones A-D), four xanthones (garciosones E-H) and three biphenyls (garciosines A-C), along with eighteen known compounds were isolated from the stems, leaves and twigs of Garcinia speciosa Wall. (Clusiaceae). Their structures were established by extensive spectroscopic analysis. For garciosines A-C, the structures were confirmed by single crystal X-ray diffraction analysis. Most of the isolated compounds were evaluated for their cytotoxic activity and anti-HIV-1 activity using the syncytium inhibition assay and HIV-1 reverse transcriptase (RT) assay. The known compounds, 4,6,3',4'-tetrahydroxy-2-methoxybenzophenone and macluraxanthone, displayed significant cytotoxic activity with the ED50 in the range of 1.85-11.76 μM. 1,5-Dihydroxyxanthone exhibited the most potent anti-HIV activity against syncytium formation with EC50 < 17.13 μM (SI > 25.28) and 2-(3,3-dimethylallyl)-1,3,7-trihydroxyxanthone was the most active compound in the HIV-1 reverse transcriptase assay with IC50 value of 58.24 μM. Structure-activity relationship of some isolated compounds were also discussed.
中文翻译:

来自藤黄的茎、叶和枝条的抗 HIV 和细胞毒性联苯、二苯甲酮和氧杂蒽酮
从 Garcinia speciosa Wall 的茎、叶和枝条中分离出 11 种以前未描述的化合物,包括四种二苯甲酮 (garciosones AD)、四种氧杂蒽酮 (garciosones EH) 和三种联苯 (garciosines AC),以及 18 种已知化合物。(Clusiaceae)。它们的结构是通过广泛的光谱分析确定的。对于garciosines AC,其结构通过单晶X射线衍射分析得到证实。使用合胞体抑制试验和 HIV-1 逆转录酶 (RT) 试验对大多数分离的化合物的细胞毒活性和抗 HIV-1 活性进行了评估。已知化合物 4,6,3',4'-四羟基-2-甲氧基二苯甲酮和 macluraxanthone 显示出显着的细胞毒活性,ED50 范围为 1.85-11.76 μM。1、5-二羟基呫吨酮对合胞体形成表现出最有效的抗 HIV 活性,EC50 < 17.13 μM (SI > 25.28),2-(3,3-二甲基烯丙基)-1,3,7-trihydroxyxanthone 是 HIV 中最活跃的化合物-1 逆转录酶测定,IC50 值为 58.24 μM。还讨论了一些分离化合物的构效关系。
更新日期:2018-03-01
中文翻译:

来自藤黄的茎、叶和枝条的抗 HIV 和细胞毒性联苯、二苯甲酮和氧杂蒽酮
从 Garcinia speciosa Wall 的茎、叶和枝条中分离出 11 种以前未描述的化合物,包括四种二苯甲酮 (garciosones AD)、四种氧杂蒽酮 (garciosones EH) 和三种联苯 (garciosines AC),以及 18 种已知化合物。(Clusiaceae)。它们的结构是通过广泛的光谱分析确定的。对于garciosines AC,其结构通过单晶X射线衍射分析得到证实。使用合胞体抑制试验和 HIV-1 逆转录酶 (RT) 试验对大多数分离的化合物的细胞毒活性和抗 HIV-1 活性进行了评估。已知化合物 4,6,3',4'-四羟基-2-甲氧基二苯甲酮和 macluraxanthone 显示出显着的细胞毒活性,ED50 范围为 1.85-11.76 μM。1、5-二羟基呫吨酮对合胞体形成表现出最有效的抗 HIV 活性,EC50 < 17.13 μM (SI > 25.28),2-(3,3-二甲基烯丙基)-1,3,7-trihydroxyxanthone 是 HIV 中最活跃的化合物-1 逆转录酶测定,IC50 值为 58.24 μM。还讨论了一些分离化合物的构效关系。


























 京公网安备 11010802027423号
京公网安备 11010802027423号