European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2018-01-02 , DOI: 10.1016/j.ejmech.2017.12.095 Seon-Mi Kim , Minhee Lee , So Young Lee , Soo-Min Lee , Eun Jeong Kim , Jae Sun Kim , Jihyae Ann , Jiyoun Lee , Jeewoo Lee
|
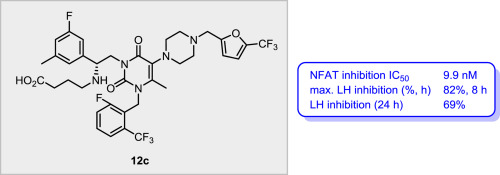
We investigated a series of uracil analogues by introducing various substituents on the phenyl ring of the N-3 aminoethyl side chain and evaluated their antagonistic activity against human gonadotropin-releasing hormone (GnRH) receptors. Analogues with substituents at the ortho or meta position demonstrated potent in vitro antagonistic activity. Specifically, the introduction of a 2-OMe group enhanced nuclear factor of activated T-cells (NFAT) inhibition up to 6-fold compared to the unsubstituted analogue. We identified compound 12c as a highly potent GnRH antagonist with moderate CYP inhibition. Compound 12c showed potent and prolonged LH suppression after a single dose was orally administered in castrated monkeys compared to a known antagonist, Elagolix. We believe that our SAR study offers useful insights to design GnRH antagonists as a potential treatment option for endometriosis.
中文翻译:

3-(2-氨基乙基)尿嘧啶衍生物作为促性腺激素释放激素(GnRH)受体拮抗剂的合成及生物学评价
我们通过在N -3氨基乙基侧链的苯环上引入各种取代基来研究一系列尿嘧啶类似物,并评估其对人促性腺激素释放激素(GnRH)受体的拮抗活性。在邻位或间位具有取代基的类似物表现出有效的体外拮抗活性。具体而言,与未取代的类似物相比,引入2-OMe基团可增强活化T细胞(NFAT)抑制的核因子,最多可提高6倍。我们将化合物12c鉴定为具有中等CYP抑制作用的高效GnRH拮抗剂。化合物12c与已知的拮抗剂Elagolix相比,在cast割的猴子中口服单次给药后,显示出有效且延长的LH抑制作用。我们认为,我们的SAR研究为将GnRH拮抗剂设计为子宫内膜异位症的潜在治疗方法提供了有用的见识。



























 京公网安备 11010802027423号
京公网安备 11010802027423号